Chemistry:Cyclodiol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | ZK-115194; Cycloestradiol; 14α,17α-Ethano-17β-estradiol; 14α,17α-Ethanoestra-1,3,5(10)-triene-3,17β-diol; 14,21-Cyclo-19-norpregna-1,3,5(10)-triene-3,17α-diol |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen |
| Pharmacokinetic data | |
| Bioavailability | 33 ± 19%[1] |
| Elimination half-life | 28.7 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
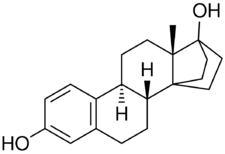
Cyclodiol (developmental code name ZK-115194; also known as 14α,17α-ethano-17β-estradiol) is a synthetic estrogen which was studied in the 1990s and was never marketed.[2][1][3] It is a derivative of estradiol with a bridge between the C14α and C17α positions.[2][1][3][4] Cyclodiol has 100% of the relative binding affinity of estradiol for the human ERα and similar transactivational capacity as estradiol at the receptor.[2] It has comparable potency to estradiol when administered by subcutaneous injection.[2] The drug shows genotoxicity similarly to estradiol.[2][4] Cyclodiol showed an absolute bioavailability of 33 ± 19% and an elimination half-life of 28.7 hours in pharmacokinetic studies in women.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Comparative pharmacokinetics of two new steroidal estrogens and ethinylestradiol in postmenopausal women". Contraception 54 (4): 235–242. October 1996. doi:10.1016/S0010-7824(96)00194-1. PMID 8922877.
- ↑ 2.0 2.1 2.2 2.3 2.4 Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. 6 December 2012. pp. 10,15,76,329,332. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA10.
- ↑ 3.0 3.1 "Studies for a genotoxic potential of some endogenous and exogenous sex steroids. I. Communication: examination for the induction of gene mutations using the Ames Salmonella/microsome test and the HGPRT test in V79 cells". Environmental and Molecular Mutagenesis 21 (3): 272–304. 1993. doi:10.1002/em.2850210311. PMID 8462531.
- ↑ 4.0 4.1 "Genotoxic potential of estrogens". Mutation Research 389 (2–3): 173–181. March 1997. doi:10.1016/S1383-5718(96)00144-1. PMID 9093381.
 |
