Chemistry:Cyclofenil
 | |
| Clinical data | |
|---|---|
| Trade names | Sexovid, others |
| Other names | Cyclophenil; F-6066; H-3452; ICI-48213; bis(p-Acetoxyphenyl)-cyclohexylidenemethane |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| Drug class | Selective estrogen receptor modulator; Progonadotropin |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 18–29 hours[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H24O4 |
| Molar mass | 364.441 g·mol−1 |
| 3D model (JSmol) | |
| |
Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women.[3][4][5][6] It is mostly no longer available.[6] The medication is taken by mouth.[7][8][9]
Side effects of cyclofenil include liver toxicity among others.[10] It is a selective estrogen receptor modulator (SERM) and hence is a mixed agonist–antagonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[8] It has antiestrogenic effects on the hypothalamic–pituitary–gonadal axis and hence can increase sex hormone production and stimulate ovulation.[8][11]
Cyclofenil was introduced for medical use in 1970.[12] It has been mostly discontinued, but remains available in a few countries, including Brazil , Italy, and Japan .[6][13][3] It has been used as a doping agent by male athletes.[8]
Medical use
Cyclofenil is used to treat menstrual disturbances and anovulatory infertility caused by insufficiency of the hypothalamic–pituitary–gonadal axis in women.[3] It has also been used to treat menopausal symptoms.[3] The medication is generally used at a dosage of 400 to 600 mg per day.[3][8][9]
Available forms
Cyclofenil has been available in the form of 100, 200, and 400 mg oral tablets.[8]
Non-medical use
Cyclofenil has been used by male athletes to increase testosterone levels.[8] It is not effective for this purpose in women.[8]
Contraindications
Cyclofenil is contraindicated during pregnancy and in those with severe liver disease and unexplained uterine bleeding.[14]
Side effects
Cyclofenil is associated with a relatively high incidence of hepatotoxicity.[10] Biochemical signs of undesirable liver changes have been observed in 35% or more of individuals and 1% of individuals experience overt hepatitis.[10]
Pharmacology
Pharmacodynamics
Cyclofenil is a SERM, or a mixed agonist and antagonist of the estrogen receptors (ERs).[8] It is described as a relatively weak/mild SERM.[8] The medication is generally less effective than other SERMs.[15] The medication is an "impeded estrogen" and is thought to work as a progonadotropin by blocking the actions of estrogens in the pituitary gland and hypothalamus, thereby disinhibiting release of the gonadotropins luteinizing hormone and follicle-stimulating hormone.[11] In men, cyclofenil can increase testosterone levels due its progonadotropic effects.[8]
Pharmacokinetics
In terms of distribution, cyclofenil acts both centrally and peripherally.[15] The elimination half-life of cyclofenil after a single 200 mg dose is 18 to 29 hours.[1][2]
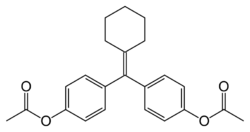
Chemistry
Cyclofenil is a nonsteroidal SERM and is closely related structurally to triphenylethylene SERMs like clomifene and tamoxifen.[9] It has been referred to as a diphenylethylene derivative, differing from triphenylethylenes only by the replacement of one of the phenyl rings with a cyclohexane ring.[16][11]
History
Cyclofenil was first introduced for medical use in 1970 under the brand name Ondogyne in France .[12] Subsequently, it was introduced throughout the world under a variety of other brand names, including its most well-known brand name Sexovid.[12]
Society and culture
Generic names
Cyclofenil is the English generic name of the drug and its INN, USAN, and BAN.[4][5][6]
Brand names
Cyclofenil has been marketed under a variety of brand names including Ciclifen, Fertodur, Gyneuro, Klofenil, Menoferil, Menopax, Neoclym, Oginex, Ondonid, Ondogyne, Rehibin, Sexadieno, Sexovar, and Sexovid.[17][12][13]
Availability
Cyclofenil remains available today only in Brazil , Italy, and Japan .[6][13][3] In the past, it has also been available in France , Germany , Mexico, Sweden, Switzerland , Turkey, and the United Kingdom .[5][12][13][3]
Regulation
Cyclofenil is included on the World Anti-Doping Agency list of illegal doping agents in sport.[18][19]
Research
Cyclofenil was investigated as a possible treatment for scleroderma in the 1980s, but was found to be ineffective.[20] Later study of its efficacy in treating Raynaud's phenomenon in people with scleroderma also found no significant benefit.[21]
References
- ↑ 1.0 1.1 Infertility: Male and Female. Churchill Livingstone. January 1993. p. 458. ISBN 978-0-443-04514-1. https://books.google.com/books?id=KCNsAAAAMAAJ.
- ↑ 2.0 2.1 Ovulation Induction and in Vitro Fertilization. Year Book Medical Publishers. 1 July 1986. p. 113. ISBN 978-0-8151-0871-9. https://books.google.com/books?id=aYVsAAAAMAAJ.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. 2009. p. 2088. ISBN 978-0-85369-840-1.
- ↑ 4.0 4.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 329–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA329.
- ↑ 5.0 5.1 5.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 284–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA284.
- ↑ 6.0 6.1 6.2 6.3 6.4 "List of 7 Menopausal Disorders Medications Compared". Drugs.com. https://www.drugs.com/international/cyclofenil.html.
- ↑ "Cyclofenil". Drug Dosage in Renal Insufficiency. Springer Science & Business Media. 6 December 2012. pp. 166–. ISBN 978-94-011-3804-8. https://books.google.com/books?id=OavnCAAAQBAJ&pg=PA166.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 "Anabolic Doping Agents". Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. 15 October 2003. pp. 555–. ISBN 978-1-59259-654-6. https://books.google.com/books?id=dwMyBwAAQBAJ&pg=PA555.
- ↑ 9.0 9.1 9.2 "Ovarian stimulation for assisted reproduction technologies". A Handbook of Intrauterine Insemination. Cambridge University Press. 31 July 1997. pp. 58–59,207. ISBN 978-0-521-58676-4. https://books.google.com/books?id=UpNScYN8B2YC&pg=PA58.
- ↑ 10.0 10.1 10.2 "Steroids and Other Hormones". Drug-Induced Hepatotoxicity. Springer Science & Business Media. 6 December 2012. pp. 565–. ISBN 978-3-642-61013-4. https://books.google.com/books?id=xZf-CAAAQBAJ&pg=PA565.
- ↑ 11.0 11.1 11.2 "Clinical Manifestations of Disorders of the Human Ovary". Physiology. Elsevier Science. 22 October 2013. pp. 209–. ISBN 978-1-4832-5975-8. https://books.google.com/books?id=4R_FAgAAQBAJ&pg=PA209.
- ↑ 12.0 12.1 12.2 12.3 12.4 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 1162–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1162.
- ↑ 13.0 13.1 13.2 13.3 "IBM Watson Health Products". http://www.micromedexsolutions.com/.
- ↑ "Ovaries". Drug Actions: Basic Principles and Therapeutic Aspects. CRC Press. 1995. pp. 294–. ISBN 978-0-8493-7774-7. https://books.google.com/books?id=IvN4mZxraMkC&pg=PA294.
- ↑ 15.0 15.1 "Excessive Vaginal Bleeding". Problems in Gynaecology. Springer Science & Business Media. 6 December 2012. pp. 105–106. ISBN 978-94-009-4125-0. https://books.google.com/books?id=q0inBgAAQBAJ&pg=PA105.
- ↑ "Oestrogens". Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. 6 December 2012. pp. 92–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA92.
- ↑ Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. 2001. p. 2397. ISBN 978-3-527-30247-5. https://books.google.com/books?id=zmpqAAAAMAAJ.
- ↑ "Hormone and metabolic modulators". Drugs in Sport. Routledge. 13 November 2014. pp. 117–. ISBN 978-1-134-70800-0. https://books.google.com/books?id=ESFWBQAAQBAJ&pg=PA117.
- ↑ Ed The Emtree Editorial Team (1 January 2004). Doping Search Guide 2004: Over 10,000 Substance Names in Reference to the 2004 WADA (World Anti-Doping Agency) List of Prohibited Substances and Methods. Elsevier. p. 82. ISBN 978-0-444-51752-4. https://books.google.com/books?id=Y9ULAQAAMAAJ.
- ↑ "Treatment of generalized systemic sclerosis". Rheum Dis Clin North Am 16 (1): 217–41. February 1990. doi:10.1016/S0889-857X(21)01050-4. PMID 2406809.
- ↑ "Cyclofenil for Raynaud's phenomenon in progressive systemic sclerosis". Cochrane Database Syst Rev 1998 (2): CD000955. 2000. doi:10.1002/14651858.CD000955. PMID 10796397.
 |
