Chemistry:Methestrol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Meprane |
| Other names | Methoestrol; Metestrol; Promethestrol; Promethoestrol; Dimethylhexestrol |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
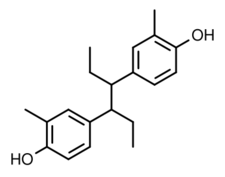
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Methestrol (INN; brand name Meprane) or methoestrol, also known as promethestrol or promethoestrol (BAN) or as dimethylhexestrol, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol which is no longer marketed.[1][2][3][4][5][6][7]
A related drug is methestrol dipropionate (or promethestrol dipropionate) (brand name Meprane Dipropionate).[8][9][10][11][12][13]
See also
References
- ↑ Dictionary of Pharmacological Agents. CRC Press. 21 November 1996. pp. 608–. ISBN 978-0-412-46630-4. https://books.google.com/books?id=Z_mfTTIApVEC&pg=PA608.
- ↑ Pharmacology for Women's Health. Jones & Bartlett Learning. 8 September 2015. pp. 640–. ISBN 978-1-284-10811-8. https://books.google.com/books?id=ofCeCgAAQBAJ&pg=PT640.
- ↑ "Preliminary clinical report on a new synthetic estrogen, meprane". American Journal of Obstetrics and Gynecology 53 (4): 678–681. April 1947. doi:10.1016/0002-9378(47)90289-5. PMID 20291241.
- ↑ "Meprane an effective estrogen for inhibition and suppression of lactation". American Journal of Obstetrics and Gynecology 54 (2): 289–295. August 1947. doi:10.1016/s0002-9378(16)39536-9. PMID 20257315.
- ↑ "Meprane in the treatment of the menopausal syndrome; an evaluation of estrogenic efficiency and toxicity". American Journal of Obstetrics and Gynecology 56 (3): 541–548. September 1948. doi:10.1016/0002-9378(48)90643-7. PMID 18877188.
- ↑ "A clinical study of a new synthetic estrogen". American Journal of Obstetrics and Gynecology 54 (2): 296–300. August 1947. doi:10.1016/s0002-9378(16)39537-0. PMID 20257316.
- ↑ "Physiology and management of the climacteric". California Medicine 71 (5): 345–348. November 1949. PMID 15390574.
- ↑ Dictionary of Pharmacological Agents. Taylor & Francis. 21 November 1996. pp. 1298–. ISBN 978-0-412-46630-4. https://books.google.com/books?id=A0THacd46ZsC&pg=PA1298.
- ↑ "Estrogens and Antiestrogenic Drug". Principles of Endocrine Pharmacology. Springer Science & Business Media. 6 December 2012. pp. 150–. ISBN 978-1-4684-5036-1. https://books.google.com/books?id=mTagBQAAQBAJ&pg=PA150.
- ↑ "Female Reprodiction – Ovary". Essential of Endocrinology and Reproductive Physiology. Allied Publishers. 1985. pp. 85–. GGKEY:HLNJ1BHUKW2. https://books.google.com/books?id=sKOwvts4me8C&pg=PA85.
- ↑ Drugs in Current Use 1958. Springer. 21 November 2013. pp. 114–. ISBN 978-3-662-40303-7. https://books.google.com/books?id=v2vwCAAAQBAJ&pg=PA114.
- ↑ Dubin (6 December 2012). Emergency Psychiatry for the House Officer. Springer Science & Business Media. pp. 155–. ISBN 978-94-011-6690-4. https://books.google.com/books?id=IwboCAAAQBAJ&pg=PT155.
- ↑ "Induction of Conditions Leading to Cancer in the Genital Tract by Estrogen Druing the Differentiation Phase of the Genital Epithelium". Hormones and Embryonic Development: Advances in The Biosciences. Elsevier Science. 22 October 2013. pp. 141–. ISBN 978-1-4831-5171-7. https://books.google.com/books?id=Xp3pAgAAQBAJ&pg=PA141.
 |
