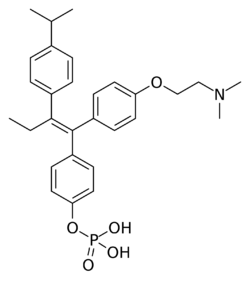
Chemistry:Miproxifene phosphate
 | |
| Clinical data | |
|---|---|
| Other names | TAT-59; Iproxifene |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C29H36NO5P |
| Molar mass | 509.583 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Miproxifene phosphate (former developmental code name TAT-59) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group[1] that was under development in Japan for the treatment of breast cancer but was abandoned and never marketed.[2][3][4][5] It reached phase III clinical trials for this indication before development was discontinued.[2][5] The drug is a phosphate ester and prodrug of miproxifene (DP-TAT-59) with improved water solubility that was better suited for clinical development.[2][3][6][7] Miproxifene has been found to be 3- to 10-fold as potent as tamoxifen in inhibiting breast cancer cell growth in in vitro models.[2][5][4] It is a derivative of afimoxifene (4-hydroxytamoxifen) in which an additional 4-isopropyl group is present in the β-phenyl ring.[8]
References
- ↑ Endocrine Therapy in Breast Cancer. CRC Press. 8 March 2002. pp. 53–. ISBN 978-0-203-90983-6. https://books.google.com/books?id=00_LBQAAQBAJ&pg=PA53.
- ↑ 2.0 2.1 2.2 2.3 "Miproxifene". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800000796.
- ↑ 3.0 3.1 Prodrugs: Challenges and Rewards. Springer Science & Business Media. 12 March 2007. pp. 168–169. ISBN 978-0-387-49782-2. https://books.google.com/books?id=qkjHxX5TgHEC&pg=PA168.
- ↑ 4.0 4.1 Cancer Chemoprevention: Volume 2: Strategies for Cancer Chemoprevention. Springer. 17 August 2008. pp. 251–. ISBN 978-1-59259-768-0. https://books.google.com/books?id=ldyg4-cem9UC&pg=PA251.
- ↑ 5.0 5.1 5.2 Nuclear Receptors as Drug Targets. John Wiley & Sons. 8 September 2008. pp. 90–. ISBN 978-3-527-62330-3. https://books.google.com/books?id=iATfLbPgRugC&pg=PA90.
- ↑ Textbook of Drug Design and Discovery, Fifth Edition. CRC Press. 19 August 2016. pp. 162–. ISBN 978-1-4987-0279-9. https://books.google.com/books?id=FcLBDAAAQBAJ&pg=PA162.
- ↑ Enzyme Technologies: Pluripotent Players in Discovering Therapeutic Agent. Wiley. 22 November 2013. pp. 166–. ISBN 978-1-118-73989-1. https://books.google.com/books?id=-nUqAgAAQBAJ&pg=PA166.
- ↑ Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. 6 December 2012. pp. 58–60. ISBN 978-3-642-58616-3. https://books.google.com/books?id=0BfrCAAAQBAJ&pg=PA58.
External links
- "Miproxifene". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800000796.
 |

