Chemistry:ZB716
 | |
| Clinical data | |
|---|---|
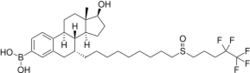
| Other names | Fulvestrant-3-boronic acid; Fulvestrant-3-boronoate; 7α-[9-[(4,4,5,5,5-Pentafluoropentyl)-sulfinyl]nonyl]-3-(dihydroxy-boryl)estra-1,3,5(10)-trien-17β-ol |
| Routes of administration | By mouth[1] |
| Drug class | Antiestrogen; Selective estrogen receptor degrader |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C32H48BF5O4S |
| Molar mass | 634.59 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 230 °C (446 °F) (decomposes) |
| |
| |
ZB716, also known as fulvestrant-3-boronic acid, is a synthetic, steroidal, orally active antiestrogen which is under development for the treatment of estrogen receptor (ER)-positive metastatic breast cancer.[1] The drug is a silent antagonist of the ERα (IC50 = 4.1 nM) as well as a selective estrogen receptor degrader (SERD).[1] It is an analogue and prodrug of fulvestrant in which the C3 hydroxyl group has been replaced with a boronic acid moiety.[1] In accordance, the two drugs have similar pharmacodynamic properties.[1] However, whereas fulvestrant is not orally active and must be administered via intramuscular injection, ZB716 is less susceptible to first-pass metabolism, and in relation to this, is orally active.[1]
A single oral dose of 8.3 mg/kg ZB716 to mice has been found to result in an over 160 ng/mL (160,000 pg/mL) maximal concentration of the drug in circulation, a level far in excess of the 15.2 ng/mL (15,200 pg/mL) concentration achieved with subcutaneous injection of fulvestrant in mice.[1] As such, not only may ZB716 be more convenient to administer in humans, it has far greater bioavailability compared to fulvestrant and hence may allow for greater systemic exposure and therapeutic benefit.[1]
ZB716 produces fulvestrant as an active metabolite in vivo in mice, with approximately 10 to 15% of the drug being converted into it.[1] As such, most of the effects are likely due to the parent drug.[1]
Clinical Development
As of December 2020, it is in a phase I clinical trial for ER+/HER2- metastatic breast cancer.[2]
See also
References
 |
