Chemistry:Benzestrol
 | |
| Clinical data | |
|---|---|
| Trade names | Chemestrogen, Ocestrol, Octestrol, Octoestrol, Octofollin |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Benzestrol (INN, BAN) (brand names Chemestrogen, Ocestrol, Octestrol, Octoestrol, Octofollin) is a synthetic nonsteroidal estrogen of the stilbestrol group which was formerly used medically but has since been discontinued.[1][2][3] The stilbestrol estrogens, the best-known of which is diethylstilbestrol (DES) were used extensively in the mid-1900s and were finally banned by the FDA due to them causing tumors in the children of women who used them.[4]
Medical uses
Benzestrol and other stilbestrol were used as synthetic estrogens in order to prevent premature births.[4] Based on the idea that premature births happened because the mother did not produce enough estrogen on her own, doctors prescribed benzestrol to mothers in order to increase their estrogen levels.
Studies have been done in the past on normal, mature and castrate female rats. Benzestrol produced the same type of estrus in the castrate rat when injected at 0.8 to 1.0 micrograms as when the rat was injected with 2.0 to 2.5 micrograms of estrone.[5] This is significant because less benzestrol could be used to produce the same effect as estrone in increasing estrogen production. It has been used in the past as a nonsteroidal estrogen antagonist.
Pharmacology
Pharmacodynamics
Benzestrol is described as a very potent estrogen.[6] It is reported to have about 130% of the relative binding affinity of estradiol for the estrogen receptors.[7]
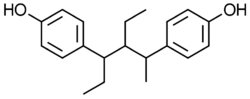
Chemistry
Benzestrol is usually grouped with the stilbestrol estrogens. However, benzestrol is technically not a stilbestrol derivative because its central chain is elongated by one carbon. In any case, it is a very close analogue of the stilbestrol estrogens.[8]
History
Benzestrol is a drug in the stilbestrol family of estrogens. These drugs were first produced in the late 1930s. Benzestrol itself was reported in 1946.[1] In 1953, experiments began on benzestrol and other stilbestrols to see if they actually helped stop premature births. This study in 1953 found that benzestrol did not in fact help stop premature births.[4] A study in 1971 found that benzestrol was the cause of a rare vaginal cancer in girls and women whose mothers had been on benzestrol while pregnant.[4]
See also
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 133–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA133.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 47–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA47.
- ↑ "Benzestrol". Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 106–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA106.
- ↑ 4.0 4.1 4.2 4.3 "DES History". U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/des/consumers/about/history.html.
- ↑ "The Effects of a New Synthetic Estrogen, Benzestrol Upon the Hematopoietic System in the Rat". Endocrinology 36 (5): 305. 1945. doi:10.1210/endo-36-5-305.
- ↑ "Sex hormones". Natural Products. Krishna Prakashan Media. 2006. pp. 288–. ISBN 978-81-87224-85-3. https://books.google.com/books?id=Zb84fVthd_cC&pg=PA288.
- ↑ "Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens". Chemistry & Biology 6 (4): 205–219. April 1999. doi:10.1016/S1074-5521(99)80037-4. PMID 10099132.
- ↑ "Therapy of the Patient in the Menopause: Endocrine Methods". The Journal of Clinical Endocrinology & Metabolism 4 (12): 597–604. 1944. doi:10.1210/jcem-4-12-597. ISSN 0021-972X.
 |
