Chemistry:Prolame
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
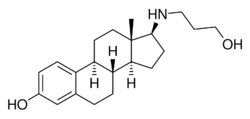
| Other names | 17β-((3-Hydroxypropyl)amino)estradiol; 17β-[(3-Hydroxypropyl)amino]estra-1,3,5(10)-trien-3-ol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H31NO2 |
| Molar mass | 329.484 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Prolame, also known as 17β-((3-hydroxypropyl)amino)estradiol, is a synthetic, steroidal estrogen and a 17β-aminoestrogen with anticoagulant effects that was first described in 1985 but was never marketed.[1][2][3][4][5]
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1025–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1025.
- ↑ "Synthesis and molecular structure of prolame, N-(3-hydroxy-1,3,5(10)-estratrien-17 beta-yl)-3-hydroxypropylamine; an amino-estrogen with prolonged anticoagulant and brief estrogenic effects". Steroids 45 (2): 151–7. 1985. doi:10.1016/0039-128x(85)90044-3. PMID 3841424.
- ↑ "The anticoagulant effect of prolame, N-(3-hydroxy-1,3,5(10)estratrien-17 beta-yl)-3-hydroxypropylamine, a novel amino-estrogen". Steroids 45 (2): 159–70. 1985. doi:10.1016/0039-128x(85)90045-5. PMID 3841425.
- ↑ "In vivo estrogen bioactivities and in vitro estrogen receptor binding and transcriptional activities of anticoagulant synthetic 17beta-aminoestrogens". J. Steroid Biochem. Mol. Biol. 73 (1–2): 59–66. 2000. doi:10.1016/s0960-0760(00)00053-4. PMID 10822025.
- ↑ Alan D. Michelson (31 December 2012). Platelets. Academic Press. pp. 314–. ISBN 978-0-12-387838-0. https://books.google.com/books?id=yF5WQdAcdfcC&pg=PA314.
 |
