Chemistry:RU-16117
 | |
| Clinical data | |
|---|---|
| Other names | 11α-Methoxyethinylestradiol; 11α-Methoxy-17α-ethynylestradiol; 11α-Methoxy-19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen; Estrogen ether |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H26O3 |
| Molar mass | 326.436 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
RU-16117 is an estrogen medication which was investigated for the potential treatment of symptoms of estrogen deficiency such as hot flashes and osteoporosis in women but was never marketed.[1] It was developed for use by mouth.[1]
Pharmacology
Pharmacodynamics
RU-16117 is an estrogen, or an agonist of the estrogen receptor (ER).[1][2] In mouse uterine tissue, it shows about 5 to 13% of the affinity of estradiol for the ER and about 1% of the estrogenic activity of estradiol.[2][3][4] Conversely, it shows no affinity for the androgen, progesterone, glucocorticoid, and mineralocorticoid receptors, nor any activities associated with interactions with these receptors.[2][5][3][4] While the association rate of RU-16117 to the ER is the same as that of moxestrol, it dissociates from the ER extremely rapidly at rates of about three times faster than estradiol and about 20 times faster than moxestrol.[1][6] This is similar to the case of estriol, which RU-16117 is described as sharing similarities with.[1][6] RU-16117 is described as a weak or partial estrogen or a mixed estrogen/antiestrogen.[1][2] It has been described as having highly active antiestrogenic activity with very weak uterotrophic activity.[7][2] However, higher doses and/or prolonged administration of RU-16117 have been reported to induce equivalent estrogenic responses relative to estradiol and moxestrol.[1][6]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 | ||
| Estriol | ? | ? | 15 | ? | ? | ? | ? | ||
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | <1 | ? | ? | ||
| Moxestrol (11β-MeO-EE) | 0.8 | <0.1 | 12 | 3.2 | <0.1 | <0.2 | <0.1 | ||
| RU-16117 (11α-MeO-EE) | 1–3 | <1 | 13 | <1 | <1 | ? | ? | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. | |||||||||
Chemistry
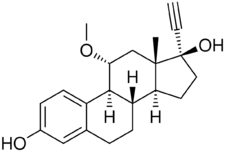
RU-16117, also known as 11α-methoxy-17α-ethynylestradiol (11α-MeO-EE) or as 11α-methoxy-17α-ethynylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrane steroid and a derivative of estradiol.[1] It is specifically a derivative of ethinylestradiol (17α-ethynylestradiol) with a methoxy group at the C11α position.[1] The compound is the C11α isomer or C11 epimer of moxestrol (11β-methoxy-17α-ethynylestradiol).[1][9]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "RU 16117, an orally active estriol-like weak estrogen". Journal of Steroid Biochemistry 20 (4B): 981–993. April 1984. doi:10.1016/0022-4731(84)90008-6. PMID 6427528.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Screening for anti-hormones by receptor studies". Proceedings of the Fourth International Congress on Hormonal Steroids: Mexico City, September 1974. Elsevier Science. 22 October 2013. pp. 618–621. ISBN 978-1-4831-4566-2. https://books.google.com/books?id=Iq0aAwAAQBAJ&pg=PA620.
- ↑ 3.0 3.1 3.2 "Steroid hormone receptors and pharmacology". Journal of Steroid Biochemistry 12: 143–157. January 1980. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- ↑ 4.0 4.1 "The relevance of interaction kinetics in determining biological response to estrogens". Endocrinology 105 (2): 509–515. August 1979. doi:10.1210/endo-105-2-509. PMID 456327.
- ↑ 5.0 5.1 "Unique steroid congeners for receptor studies". Cancer Research 38 (11 Pt 2): 4186–4198. November 1978. PMID 359134. http://cancerres.aacrjournals.org/content/38/11_Part_2/4186.short.
- ↑ 6.0 6.1 6.2 "The Mechanism of Action of Anti-hormones". Receptors: Proceedings of the 7th International Congress of Pharmacology, Paris, 1978. Elsevier. 26 January 2016. pp. 261–,266–267,274. ISBN 978-1-4831-5796-2. https://books.google.com/books?id=RAIlBQAAQBAJ&pg=PA261.
- ↑ "High inhibitory activity of a new antiestrogen, RU 16117 (11alpha-methoxy ethinyl estradiol), on the development of dimethylbenz(a)anthracene-induced mammary tumors". Cancer Research 37 (1): 76–81. January 1977. PMID 187338.
- ↑ "Towards the mapping of the progesterone and androgen receptors". Journal of Steroid Biochemistry 27 (1–3): 255–269. 1987. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ "Influence of rat estradiol binding protein (EBP) on estrogen binding to its receptor and on induced biological responses". Development of Responsiveness to Steroid Hormones: Advances in the Biosciences. Elsevier Science. 22 October 2013. pp. 61–. ISBN 978-1-4831-5308-7. https://books.google.com/books?id=rtXWAgAAQBAJ&pg=PA61.
 |

