Chemistry:Equilenin
 | |
| Clinical data | |
|---|---|
| Other names | 6,8-Didehydroestrone; Estra-1,3,5(10),6,8-pentaen-3-ol-17-one |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H18O2 |
| Molar mass | 266.340 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
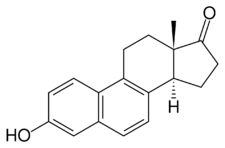
Equilenin, also known as 6,8-didehydroestrone, as well as estra-1,3,5(10),6,8-pentaen-3-ol-17-one, is a naturally occurring steroidal estrogen obtained from the urine of pregnant mares.[1][2] It is used as one of the components in conjugated estrogens (brand name Premarin).[2] It was the first complex natural product to be fully synthesized, in work reported by 1940 by Bachmann and Wilds.[3]
Chemistry
Synthesis
Total synthesis
The synthesis developed by the Bachmann group started from Butenand's ketone[4] – the 7-methoxy structural analog of 1,2,3,4-tetrahydrophenanthren-1-one[5] – and which can be readily prepared from 1,6-Cleve's acid.[6] The approach was based on well-established transformations like the Claisen condensation, the Reformatsky reaction, the Arndt–Eistert reaction, and the Dieckmann condensation.[3] Nicolaou described this preparation as ending the era preceding the post-World War II work of Robert Burns Woodward that introduced enantioselective synthesis;[4] in this synthesis, a mixture of stereoisomers were prepared and then resolved,[6] and the choice of target was partly because of the existence of only two chiral carbons and hence only four stereoisomers.[5]
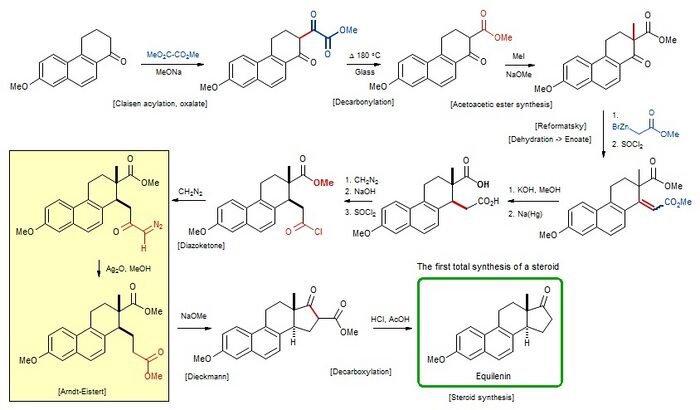
The overall yield of the synthesis was 2.7% based on a twenty-step process starting from Cleve's acid.[6]
See also
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 494–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA298.
- ↑ 2.0 2.1 Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. 28 March 2012. pp. 751–. ISBN 978-1-4511-4847-3. https://books.google.com/books?id=KZLubBxJEwEC&pg=PA751.
- ↑ 3.0 3.1 "The Total Synthesis of the Sex Hormone Equilenin and Its Stereoisomers". J. Am. Chem. Soc. 62 (4): 824–839. 1940. doi:10.1021/ja01861a036.
- ↑ 4.0 4.1 "The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century". Angewandte Chemie 39 (1): 44–122. January 2000. doi:10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L. PMID 10649349. http://yxzx.zjnu.edu.cn/lunwen/8.pdf. Retrieved 2017-07-22.
- ↑ 5.0 5.1 "The Total Synthesis of the Sex Hormone Equilenin". J. Am. Chem. Soc. 61 (4): 974–975. 1939. doi:10.1021/ja01873a513.
- ↑ 6.0 6.1 6.2 "Steroids". Natural Products Chemistry. 1. Academic Press. 1974. pp. 421–545. ISBN 9781483218861. https://books.google.com/books?id=PKb-BAAAQBAJ&pg=PA491.
 |
