(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
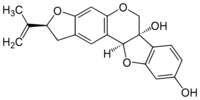
Glyceollin III
|
| Names
|
Preferred IUPAC name
(2S,6aS,11aS)-2-(Prop-1-en-2-yl)-1,2-dihydro-6H-[1]benzofuro[3,2-c]furo[3,2-g][1]benzopyran-6a,9(11aH)-diol |
| Identifiers
|
|
|
|
|
|
|
| ChEBI
|
|
| ChEMBL
|
|
| ChemSpider
|
|
| KEGG
|
|
|
|
|
| UNII
|
|
InChI=1S/C20H18O5/c1-10(2)15-6-11-5-13-17(8-16(11)24-15)23-9-20(22)14-4-3-12(21)7-18(14)25-19(13)20/h3-5,7-8,15,19,21-22H,1,6,9H2,2H3/t15-,19-,20+/m0/s1 Key: MIYTVBARXCVVHZ-RYGJVYDSSA-N
|
CC(=C)[C@@H]1Cc2cc3c(cc2O1)OC[C@@]4([C@H]3Oc5c4ccc(c5)O)O
|
| Properties
|
|
|
C20H18O5
|
| Molar mass
|
338.35 g/mol
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
| Infobox references
|
|
|
|
Tracking categories (test):
Glyceollin III is a glyceollin, a type of pterocarpan, found in the soybean (Glycine max).[1][2] It has an antiestrogenic effect.[3] In soil, it has an antifungal activity against Aspergillus sojae.[4]
References
- ↑ Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy. M. Carla Zimmermann, Syreeta L. Tilghman, Stephen M. Boué, Virgilio A. Salvo, Steven Elliott, K. Y. Williams, Elena V. Skripnikova, Hasina Ashe, Florastina Payton-Stewart, Lyndsay Vanhoy-Rhodes, Juan Pablo Fonseca, Cynthia Corbitt, Bridgette M. Collins-Burow, Melanie H. Howell, Michelle Lacey, Betty Y. Shih, Carol Carter-Wientjes, Thomas E. Cleveland, John A. McLachlan, Thomas E. Wiese, Barbara S. Beckman and Matthew E. Burow, JPET January 2010 vol. 332 no. 1, doi:10.1124/jpet.109.160382
- ↑ Biosynthesis of glyceollins I, II and III in soybean. Stephen W. Banks and Paul M. Dewick, Phytochemistry, Volume 22, Issue 12, 1983, pp. 2729-2733, doi:10.1016/S0031-9422(00)97682-9
- ↑ Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Salvo Virgilo A., Boue Stephen M., Fonseca Juan P., Elliott Steven, Corbitt Cynthia, Collins-Burow Bridgette M., Curiel Tyler J., Srivastav Sudesh K., Shih Betty Y., Carter-Wientjes Carol, Wood Charles E., Erhardt Paulw., Beckman Barbara S., McLachlan John A., Cleveland Thomas E. and Burow Matthew E., Clinical Cancer Research, 2006, vol. 12, no23, pp. 7159-7164
- ↑ Antifungal Activity of Glyceollins Isolated from Soybean Elicited with Aspergillus sojae. Hyo Jung Kim, Hwa-Jin Suh, Choong Hwan Lee, Jeong Hwan Kim, Sun Chul Kang, Sunmin Park and Jong-Sang Kim, J. Agric. Food Chem., 2010, 58 (17), pp. 9483–9487, doi:10.1021/jf101694t
|
|---|
| ER | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, [[Chemistry:Epicateepicatechin, Chemistry:Equol|equol]], formononetin, glabrene, glabridin, Genistein|genistein]], genistin, glycitein, kaempferol, Chemistry:Liquiritigenin
|
|---|
Mixed
(SERMs) | |
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|
| GPER | |
|---|
|
 | Original source: https://en.wikipedia.org/wiki/Glyceollin III. Read more |



