Chemistry:Propylpyrazoletriol
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
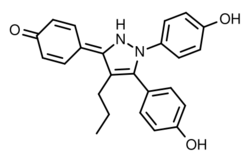
| Formula | C24H22N2O3 |
| Molar mass | 386.451 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Propylpyrazoletriol (PPT) is a synthetic, nonsteroidal agonist of ERα with 400-fold selectivity over ERβ[1] that is used widely in scientific research to study the function of ERα.[2][3][4] Though originally thought to be highly selective for ERα, PPT has subsequently been found to also act as an agonist of the GPER (GPR30).[5]
See also
References
- ↑ "Untangling the Estrogen Receptor Web: Tools to Selectively Study Estrogen‐Binding Receptors". Nuclear Receptors as Drug Targets. Methods and Principles in Medicinal Chemistry. John Wiley & Sons. 8 September 2008. pp. 47–64 (50). doi:10.1002/9783527623297.ch3. ISBN 978-3-527-62330-3.
- ↑ "Female sexual behavior: Hormonal Priming and Control". Knobil and Neill's Physiology of Reproduction: Two-Volume Set. 2. Academic Press. 15 November 2014. pp. 2287-2370 (2311). ISBN 978-0-12-397769-4. https://www.researchgate.net/publication/282594468.
- ↑ "Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β". Journal of Neurochemistry 115 (5): 1277–87. December 2010. doi:10.1111/j.1471-4159.2010.07038.x. PMID 20977477.
- ↑ Mann MK (2008). Synthesis of Non-steroidal Estrogen Receptor Proteolysis Targeting Chimeric Molecules (PROTACS) (Ph.D. thesis). University of Illinois at Urbana-Champaign. pp. 11–.
- ↑ "Estrogen biology: new insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology 389 (1–2): 71–83. May 2014. doi:10.1016/j.mce.2014.02.002. PMID 24530924.
 |

