Chemistry:16α-Iodo-E2
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H23IO2 |
| Molar mass | 398.284 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
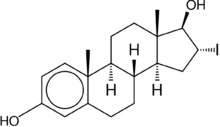
16α-Iodo-E2, or 16α-iodoestradiol, is a synthetic, steroidal, potent estrogen with slight preference for the ERα over the ERβ that is used in scientific research.[1][2] The KD of 16α-iodo-E2 for the ERα is 0.6 nM and for the ERβ is 0.24 nM, a 4-fold difference in affinity, whereas estradiol is considered to have similar affinity for the two receptor subtypes.[2] Unlike the case of the much weaker estriol (16α-hydroxyestradiol), 16α-iodo-E2 is considered to be equipotent with estradiol in terms of estrogenic activity.[3] Radiolabeled [16α-125I]iodo-E2 has been employed in imaging to study the estrogen receptor.[4]
See also
References
- ↑ "Structure-based design of estrogen receptor-beta selective ligands". J. Am. Chem. Soc. 126 (46): 15106–19. 2004. doi:10.1021/ja047633o. PMID 15548008.
- ↑ 2.0 2.1 "Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer". Curr Cancer Drug Targets 8 (5): 367–77. 2008. doi:10.2174/156800908785133150. PMID 18690843.
- ↑ "The molecular structure of 16 alpha-iodo-17 beta-estradiol, a high affinity ligand for the estrogen receptor". J. Steroid Biochem. 25 (5A): 615–8. 1986. doi:10.1016/0022-4731(86)90002-6. PMID 3795941.
- ↑ "Radiolabeled steroidal estrogens in cancer research". Steroids 58 (6): 245–59. 1993. doi:10.1016/0039-128x(93)90069-y. PMID 8212070.
 |

