Short description: Class of chemical compounds
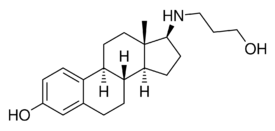
17β-Aminoestrogens are a group of synthetic, steroidal estrogens derived from estradiol which have an amine substitution in place of the hydroxyl group at the C17β position.[1][2] They are estrogenic similarly,[1] but, unlike estradiol, show sustained anticoagulant activity that appears to be mediated by non-genomic mechanisms.[2] As such, it is thought that they may have a reduced risk of venous thromboembolism.[2] The 17β-aminoestrogens include the base or parent estrogen aminoestradiol (AE2)[3] and the extended-chain derivatives butolame, hexolame, pentolame, prodiame, and prolame.[1][2] They are a homologous series of steroids.[4]
References
- ↑ 1.0 1.1 1.2 "Anticoagulant and estrogenic effects of two new 17 beta-aminoestrogens, butolame [17 beta-(4-hydroxy-1-butylamino)-1,3,5(10)-estratrien-3-ol] and pentolame [17 beta-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol]". Steroids 58 (10): 457–61. 1993. doi:10.1016/0039-128x(93)90002-5. PMID 8256254.
- ↑ 2.0 2.1 2.2 2.3 "The antithrombotic effect of the aminoestrogen prolame (N-(3-hydroxy-1,3,5(10)-estratrien-17B-YL)-3-hydroxypropylamine) is linked to an increase in nitric oxide production by platelets and endothelial cells". Atherosclerosis 208 (1): 62–8. 2010. doi:10.1016/j.atherosclerosis.2009.06.017. PMID 19615684.
- ↑ "In vivo profile of the anticoagulant effect of 17ß-amino-1,3,5(10)estratrien-3-ol". Eur. J. Pharmacol. 700 (1–3): 210–6. 2013. doi:10.1016/j.ejphar.2012.12.030. PMID 23305838.
- ↑ "Comparison of the time course of anticoagulant and estrogenic effects of prolame, butolame, pentolame and hexolame, a homologous series of 17 beta-amino estrogens". Proc. West. Pharmacol. Soc. 36: 143–7. 1993. PMID 8378368.
|
|---|
| ER | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, [[Chemistry:Epicateepicatechin, Chemistry:Equol|equol]], formononetin, glabrene, glabridin, Genistein|genistein]], genistin, glycitein, kaempferol, Chemistry:Liquiritigenin
|
|---|
Mixed
(SERMs) | |
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|
| GPER | |
|---|
|
 | Original source: https://en.wikipedia.org/wiki/17β-Aminoestrogen. Read more |