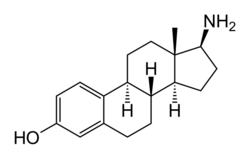
Chemistry:Aminoestradiol
 | |
| Clinical data | |
|---|---|
| Other names | AE2; 17βAE2; 17β-Aminoestra-1,3,5(10)-trien-3-ol; 3-Hydroxy-17β-aminoestra-1,3,5(10)-triene |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H25NO |
| Molar mass | 271.404 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Aminoestradiol (AE2), also known as 17β-aminoestradiol (17βAE2) or as 17β-aminoestra-1,3,5(10)-trien-3-ol, is a synthetic, steroidal estrogen and a 17β-aminoestrogen with anticoagulant effects that was never marketed.[1][2][3][4][5] It is an analogue of estradiol in which the C17β hydroxyl group has been replaced with an amine group.[1][2] AE2 has profoundly reduced estrogenic potency compared to estradiol; its EC50 for activation of the ERα was found to be 1.82 μM, whereas that of estradiol was 2.14 nM (relative potency 0.12 for AE2 versus 100 for estradiol, or roughly a 1,000-fold difference).[2] It binds with low relative affinity to both the ERα and ERβ and has estrogenic activity that is greatly mediated through the ERβ and to a lesser extent through the ERα.[2]
References
- ↑ 1.0 1.1 "In vivo profile of the anticoagulant effect of 17ß-amino-1,3,5(10)estratrien-3-ol". Eur. J. Pharmacol. 700 (1–3): 210–6. 2013. doi:10.1016/j.ejphar.2012.12.030. PMID 23305838.
- ↑ 2.0 2.1 2.2 2.3 "In vivo and in vitro estrogenic profile of 17β-amino-1,3,5(10)estratrien-3-ol". J. Steroid Biochem. Mol. Biol. 147: 40–7. 2015. doi:10.1016/j.jsbmb.2014.11.019. PMID 25448750.
- ↑ "Synthesis of new amino acid and peptide derivatives of estradiol and their binding affinities for the estrogen receptor". Steroids 58 (1): 35–9. 1993. doi:10.1016/0039-128x(93)90015-f. PMID 8430443.
- ↑ "A multiplexed screening method for agonists and antagonists of the estrogen receptor protein". Anal Bioanal Chem 403 (5): 1373–84. 2012. doi:10.1007/s00216-012-5933-7. PMID 22453607.
- ↑ "A comparative structural study of the steroid epimers: 17 beta-amino-1,3,5(10)-estratrien-3-ol, 17 alpha-amino-1,3,5(10)-estratrien-3-ol, and some derivatives by 1H NMR, and x-ray diffraction analysis". Steroids 63 (11): 556–64. 1998. doi:10.1016/s0039-128x(98)00063-4. PMID 9830681.
 |

