Chemistry:Tetrabenazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xenazine, Xentra, Nitoman, others |
| Other names | Ro-1-9569 |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low, extensive first pass effect |
| Protein binding | 82–85% |
| Metabolism | Liver (CYP2D6-mediated) |
| Elimination half-life | 10 hours parent compound (2 to 8 hours active metabolites)[2] |
| Excretion | Kidney (~75%) and fecal (7–16%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H27NO3 |
| Molar mass | 317.429 g·mol−1 |
| 3D model (JSmol) | |
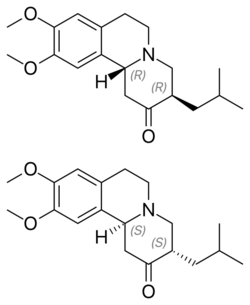
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Tetrabenazine is a drug for the symptomatic treatment of hyperkinetic movement disorders. It is sold under the brand names Nitoman and Xenazine among others. On August 15, 2008, the U.S. Food and Drug Administration approved the use of tetrabenazine to treat chorea associated with Huntington's disease. Although other drugs had been used "off label," tetrabenazine was the first approved treatment for Huntington's disease in the U.S.[4] The compound has been known since the 1950s.
Medical uses
Tetrabenazine is used as a treatment, but not as a cure, for hyperkinetic disorders such as:[5][6]
- Huntington's disease – specifically, the chorea associated with it
- Tourette syndrome and other tic disorders
- Tardive dyskinesia,[7] a serious and sometimes irreversible side effect of long-term use of many antipsychotics, mainly typical antipsychotics
- Hemiballismus, spontaneous flinging limb movements due to contra-lateral subthalamic nucleus damage
Tetrabenazine has been used as an antipsychotic in the treatment of schizophrenia, both in the past[8][9][10][11][12][13][14][15] and in modern times.[16][17][18]
Side effects
The most common adverse reactions, which have occurred in at least 10% of subjects in studies and at least 5% greater than in subjects who received placebo, have been: sedation or somnolence, fatigue, insomnia, depression, suicidal thoughts, akathisia, anxiety and nausea.[3]
Warnings
There is a boxed warning associated with the use of tetrabenazine:[3]
- Increases the risk of depression and suicidal thoughts and behavior in patients with Huntington's disease
- Balance risks of depression and suicidality with the clinical need for control of chorea when considering the use of tetrabenazine
- Monitor patients for emergence or worsening of depression, suicidality or unusual changes in behavior
- Inform patients, caregivers and families of the risk of depression and suicidality and instruct to report behaviours of concern promptly to the treating physician
- Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation
- Tetrabenazine is contraindicated in patients who are actively suicidal and in patients with untreated or inadequately treated depression
Pharmacology
The precise mechanism of action of tetrabenazine is unknown. Its anti-chorea effect is believed to be due to a reversible depletion of monoamines such as dopamine, serotonin, norepinephrine, and histamine from nerve terminals. Tetrabenazine reversibly inhibits vesicular monoamine transporter 2, resulting in decreased uptake of monoamines into synaptic vesicles, as well as depletion of monoamine storage.[3]
See also
References
- ↑ "TETRABENAZINE RAN/TETRABENAZINE SUN/TETRABENAZINE RBX (Sun Pharma ANZ Pty Ltd)". https://www.tga.gov.au/resources/prescription-medicines-registrations/tetrabenazine-rantetrabenazine-suntetrabenazine-rbx-sun-pharma-anz-pty-ltd.
- ↑ "Tetrabenazine (Xenazine), An FDA-Approved Treatment Option For Huntington's Disease-Related Chorea". P & T 33 (12): 690–694. December 2008. PMID 19750050.
- ↑ 3.0 3.1 3.2 3.3 "Xenazine (tetrabenazine) Tablets, for Oral Use. Full Prescribing Information. Revised: 6/2015". H. Lundbeck A/S. http://www.lundbeck.com/upload/us/files/pdf/Products/Xenazine_PI_US_EN.pdf.
- ↑ 1st US drug for Huntington's disease wins approval [|permanent dead link|dead link}}]
- ↑ "Long-term effects of tetrabenazine in hyperkinetic movement disorders". Neurology 48 (2): 358–62. 1997. doi:10.1212/wnl.48.2.358. PMID 9040721.
- ↑ "Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders". Movement Disorders 22 (2): 193–7. January 2007. doi:10.1002/mds.21222. PMID 17133512.
- ↑ "Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol". American Journal of Psychiatry 156 (8): 1279–81. August 1999. doi:10.1176/ajp.156.8.1279. PMID 10450276. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=10450276.
- ↑ "Clinical comparison of tetrabenazine (Ro 1-9569), reserpine and placebo in chronic schizophrenics". Diseases of the Nervous System 21(3)Suppl (3 Suppl): 120–123. March 1960. PMID 13832091.
- ↑ "[First clinical experiences with tetrabenazine]" (in Italian). Rassegna di Studi Psichiatrici 49: 450–460. 1960. PMID 13745210.
- ↑ "[On the pharmacotherapy of psychoses: clinical research on tetrabenazine]" (in German). Psychiatria et Neurologia 140: 23–29. July 1960. doi:10.1159/000131224. PMID 13748124.
- ↑ "A comparison of tetrabenazine and chlorpromazine in chronic schizophrenia". The Journal of Mental Science 107 (447): 287–293. March 1961. doi:10.1192/bjp.107.447.287. PMID 13684728.
- ↑ "[Clinical, biological and electroencephalographic study of the action of tetrabenazine (Ro 956) in various chronic psychoses]" (in French). Annales Médico-Psychologiques 120 (1): 115–119. January 1962. PMID 13874731.
- ↑ "[Use of a new neuroleptic: tetrabenazine. Clinical, biological and electroencephalographic results]" (in French). Annales Médico-Psychologiques 120 (1): 106–115. January 1962. PMID 14453492.
- ↑ "Tetrabenazine (Nitoman) in the treatment of psychoses. With a discussion on the central mode of action of tetrabenazine and reserpine.". Acta Psychiatrica Scandinavica 39: SUPPL170:1–SUPPL17109. 1963. PMID 14081399.
- ↑ "[Therapy of schizophrenia with tetrabenazine]" (in Japanese). Nihon Rinsho. Japanese Journal of Clinical Medicine 24 (7): 1360–1364. July 1966. PMID 6007641.
- ↑ "Neurotransmitter depleter tetrabenazine; potential candidate for schizophrenia treatment?". Schizophrenia Research 96 (1–3): 267–268. November 2007. doi:10.1016/j.schres.2007.07.010. PMID 17683910.
- ↑ "Tetrabenazine augmentation in treatment-resistant schizophrenia: a 12-week, double-blind, placebo-controlled trial". Journal of Clinical Psychopharmacology 32 (1): 95–99. February 2012. doi:10.1097/JCP.0b013e31823f913e. PMID 22198452.
- ↑ "Tetrabenazine: Spotlight on Drug Review". Annals of Neurosciences 23 (3): 176–185. September 2016. doi:10.1159/000449184. PMID 27721587.
External links
- Xenazine prescribing information FDA
- NIMH Repository data sheet
- "Tetrabenazine" from HOPES: Huntington's Disease Outreach Project for Education at Stanford
- Detailed monograph on tetrabenazine on rxmed.com
- Information on tetrabenazine from netdoctor.co.uk
 |

