Chemistry:Cyclotriol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | ZK-136295; Cycloestriol; 14α,17α-Ethanoestriol; 14α,17α-Ethanoestra-1,3,5(10)-triene-3,16α,17β-triol; 14,21-Cyclo-19-norpregna-1,3,5(10)-triene-3,16α,17α-triol |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen |
| Pharmacokinetic data | |
| Bioavailability | 40%[1] |
| Elimination half-life | 12.3 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H26O3 |
| Molar mass | 314.425 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
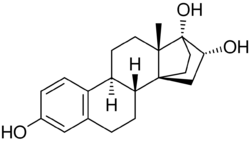
Cyclotriol (developmental code name ZK-136295; also known as 14α,17α-ethanoestriol) is a synthetic estrogen which was studied in the 1990s and was never marketed.[2][1][3][4] It is a derivative of estriol with a bridge between the C14α and C17α positions.[2][1][3][5] The drug has 40% of the relative binding affinity of estradiol for the human ERα.[2] It showed an absolute bioavailability of 40% with high interindividual variability and an elimination half-life of 12.3 hours in pharmacokinetic studies in women.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Comparative pharmacokinetics of two new steroidal estrogens and ethinylestradiol in postmenopausal women". Contraception 54 (4): 235–242. October 1996. doi:10.1016/S0010-7824(96)00194-1. PMID 8922877.
- ↑ 2.0 2.1 2.2 Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. 6 December 2012. pp. 10,15,76,331–332. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA10.
- ↑ 3.0 3.1 "Studies for a genotoxic potential of some endogenous and exogenous sex steroids. I. Communication: examination for the induction of gene mutations using the Ames Salmonella/microsome test and the HGPRT test in V79 cells". Environmental and Molecular Mutagenesis 21 (3): 272–304. 1993. doi:10.1002/em.2850210311. PMID 8462531.
- ↑ "Studies for a genotoxic potential of some endogenous and exogenous sex steroids. II. Communication: examination for the induction of cytogenetic damage using the chromosomal aberration assay on human lymphocytes in vitro and the mouse bone marrow micronucleus test in vivo". Environmental and Molecular Mutagenesis 28 (2): 133–144. 1996. doi:10.1002/(SICI)1098-2280(1996)28:2<133::AID-EM10>3.0.CO;2-G. PMID 8844995.
- ↑ "Genotoxic potential of estrogens". Mutation Research 389 (2–3): 173–181. March 1997. doi:10.1016/S1383-5718(96)00144-1. PMID 9093381.
 |

