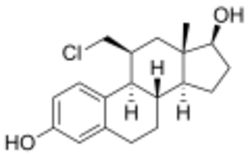
Chemistry:11β-Chloromethylestradiol
 | |
| Clinical data | |
|---|---|
| Other names | 11β-CME2; CME; ORG-4333; 11β-(Chloromethyl)estra-1,3,5(10)-trien-3,17β-diol |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C19H25ClO2 |
| Molar mass | 320.86 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
11β-Chloromethylestradiol (11β-CME2; developmental code name ORG-4333) is a synthetic steroidal estrogen which was never marketed.[1][2][3][4][5][6] It has very high affinity for the estrogen receptor and dissociates from it relatively slowly.[1][2][4][5][7][8] It was originally thought that 11β-CME2 might be a covalent ligand of the estrogen receptors, but its binding was subsequently shown to be fully reversible.[3] The relative binding affinity of 11β-CME2 for the estrogen receptors ranges from 230 to 3,320% of that of estradiol depending on the study.[5][9][7][8][10][11] 11β-CME2 also has about 14% of the relative binding affinity of estradiol for sex hormone-binding globulin (SHBG).[11] The compound has been developed as a radiolabel for the ERs.[3][4]
See also
References
- ↑ 1.0 1.1 "Equilibrium binding analysis of estrogen agonists and antagonists: relation to the activation of the estrogen receptor". Pathologie-Biologie 39 (1): 59–69. January 1991. PMID 2011412.
- ↑ 2.0 2.1 "11 beta-chloromethyl-[3H]estradiol-17 beta: a very high affinity, reversible ligand for the estrogen receptor". Journal of Steroid Biochemistry 28 (4): 361–370. October 1987. doi:10.1016/0022-4731(87)91052-1. PMID 3669657.
- ↑ 3.0 3.1 3.2 "A comparison of 11 beta-chloromethylestradiol and tamoxifen aziridine as affinity labeling reagents for estrogen receptors". Steroids 51 (5–6): 499–518. 1988. doi:10.1016/0039-128x(88)90047-5. PMID 3242173.
- ↑ 4.0 4.1 4.2 "The synthesis and study of some potential affinity labeling reagents for estrogen receptors". Steroids 38 (5): 537–555. November 1981. doi:10.1016/0039-128x(81)90053-2. PMID 7324085.
- ↑ 5.0 5.1 5.2 "Biological activity and receptor binding of a strongly interacting estrogen in human breast cancer cells". Cancer Research 44 (6): 2302–2308. June 1984. PMID 6547074. https://cancerres.aacrjournals.org/content/44/6/2302.short.
- ↑ "The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants". Environmental Health Perspectives 103 (Suppl 7): 113–122. October 1995. doi:10.1289/ehp.95103s7113. PMID 8593856.
- ↑ 7.0 7.1 "Reversible, positive cooperative interaction of 11 beta-chloromethyl-[3H]estradiol-17 beta with the calf uterine estrogen receptor". Journal of Steroid Biochemistry 33 (5): 859–865. November 1989. doi:10.1016/0022-4731(89)90233-1. PMID 2601330.
- ↑ 8.0 8.1 "Interactions of exogenous endocrine active substances with nuclear receptors". Pure and Applied Chemistry 75 (11–12): 1797–1817. 2003. doi:10.1351/pac200375111797. ISSN 1365-3075.
- ↑ "Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens". Chemistry & Biology 6 (4): 205–219. April 1999. doi:10.1016/S1074-5521(99)80037-4. PMID 10099132.
- ↑ "CoMSIA and docking study of rhenium based estrogen receptor ligand analogs". Steroids 72 (3): 247–260. March 2007. doi:10.1016/j.steroids.2006.11.011. PMID 17280694.
- ↑ 11.0 11.1 "Synthesis, structure and biological properties of Z-17alpha-(2-iodovinyl)-11beta-chloromethyl estradiol-17beta (Z-CMIV), a high affinity ligand for the characterization of estrogen receptor-positive tumors". The Journal of Steroid Biochemistry and Molecular Biology 59 (1): 103–117. September 1996. doi:10.1016/s0960-0760(96)00007-6. PMID 9009243.
 |

