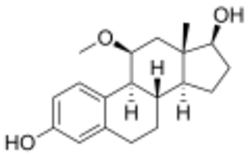
Chemistry:11β-Methoxyestradiol
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | 11β-MeOE2; 11βOMeEST; RU-2504; 11β-Methoxyestra-1,3,5(10)-triene-3,17β-diol |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H26O3 |
| Molar mass | 302.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
11β-Methoxyestradiol (11β-MeOE2; developmental code name RU-2504) is a synthetic steroidal estrogen which was never marketed.[1][2][3] It has about 86% of the relative binding affinity of estradiol for the estrogen receptor.[1][2] 11β-MeOE2 is structurally related to moxestrol (11β-methoxy-17α-ethynylestradiol).[3][2] 11β-MeOE2 and moxestrol are substantially more potent than their non-methoxylated analogues (estradiol and ethinylestradiol, respectively) in mice.[3]
See also
References
- ↑ 1.0 1.1 "CoMSIA and docking study of rhenium based estrogen receptor ligand analogs". Steroids 72 (3): 247–260. March 2007. doi:10.1016/j.steroids.2006.11.011. PMID 17280694.
- ↑ 2.0 2.1 2.2 "Interactions of exogenous endocrine active substances with nuclear receptors". Pure and Applied Chemistry 75 (11–12): 1797–1817. 2003. doi:10.1351/pac200375111797. ISSN 1365-3075.
- ↑ 3.0 3.1 3.2 "Synthèse et activité utérotrophique des 11β-méthoxy estradiol 11β-méthoxy estriol et 11β-méthoxy 17α-éthynyl estradiol". Chimica Therapeutica 8 (4): 451–454. 1973. ISSN 0009-4374. "The prepn. and estrogenic activity of the 11β-methoxylated derivs. of estradiol, estriol, and 17α-ethynylestradiol were described. When administered orally to mice, the 3 compds. were from 10 to 1000 times more active than the corresponding nonmethoxylated estrogens.".
 |
