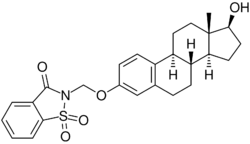
Chemistry:Estradiol 3-saccharinylmethyl ether
 | |
| Clinical data | |
|---|---|
| Other names | E2SME; 3-O-(Saccharinylmethyl)-17β-estradiol; 3-O-(Saccharinylmethyl)estra-1,3,5(10)-triene-3,17β-diol |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H29NO5S |
| Molar mass | 467.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estradiol 3-saccharinylmethyl ether (E2SME), also known as 3-O-(saccharinylmethyl)-17β-estradiol, is a synthetic estrogen and estrogen ether – specifically, the C3 saccharinylmethyl ether of estradiol – which was described in the mid-1990s and was never marketed.[2][1][3][4] It is a prodrug of estradiol and appears to be partially protected from first-pass metabolism in the liver and intestines with oral administration, showing greatly improved oral potency compared to estradiol.[1][3][4]
E2SME has been found to be 9-fold as potent as estradiol by the oral route in rats.[1][4] Similarly, its bioavailability (16%) was 5-fold greater than that of estradiol via the oral route in rats, and the elimination half-life of released estradiol was 5- to 7-fold longer than that of regular estradiol.[1][3][4] Conversely, when E2SME and estradiol were given intravenously in rats, there was no difference between them in terms of potency.[1] In vitro studies revealed that E2SME is not hydrolyzed to estradiol enzymatically but rather is hydrolyzed chemically in biological media such as plasma, apparently dependent on the concentration of protein.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "A prodrug approach to increasing the oral potency of a phenolic drug. Part 2. Pharmacodynamics and preliminary bioavailability of an orally administered O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences 84 (2): 174–178. February 1995. doi:10.1002/jps.2600840210. PMID 7738796.
- ↑ "A prodrug approach to increasing the oral potency of a phenolic drug. 1. Synthesis, characterization, and stability of an O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences 83 (10): 1477–1481. October 1994. doi:10.1002/jps.2600831022. PMID 7884673.
- ↑ 3.0 3.1 3.2 "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. 6 December 2012. pp. 263–. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA263.
- ↑ 4.0 4.1 4.2 4.3 "Prodrugs to Reduce Presystemic Metabolism". Prodrugs. Biotechnology: Pharmaceutical Aspects. V. Springer. 2007. pp. 339–355. doi:10.1007/978-0-387-49785-3_8. ISBN 978-0-387-49782-2.
 |

