Biology:TRPV
| Transient receptor potential (TRP) ion channel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
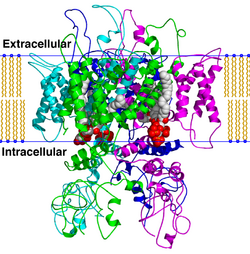 Homology model of the TRPV1 ion channel tetramer (where the monomers are individually colored cyan, green, blue, and magenta respective) imbedded in a cartoon representation of a lipid bilayer. PIP2 signaling ligands are represented by space-filling models (carbon = white, oxygen = red, phosphorus = orange).[1] | |||||||||
| Identifiers | |||||||||
| Symbol | TRP | ||||||||
| Pfam | PF06011 | ||||||||
| InterPro | IPR010308 | ||||||||
| |||||||||
TRPV is a family of transient receptor potential cation channels (TRP channels) in animals. All TRPVs are highly calcium selective.
TRP channels are a large group of ion channels consisting of six protein families, located mostly on the plasma membrane of numerous human and animal cell types, and in some fungi.[2] TRP channels were initially discovered in the trp mutant strain of the fruit fly Drosophila [3] that displayed transient elevation of potential in response to light stimuli, and were therefore named "transient receptor potential" channels.[4] The name now refers only to a family of proteins with similar structure and function, not to the mechanism of their activation. Later, TRP channels were found in vertebrates where they are ubiquitously expressed in many cell types and tissues. There are about 28 TRP channels that share some structural similarity to each other.[5] These are grouped into two broad groups: group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanilloid), TRPM ("M" for melastatin), TRPN and TRPA. In group 2 there are TRPP ("P" for polycystic) and TRPML ("ML" for mucolipin).
Structure
Functional TRPV ion channels are tetrameric in structure and are either homo-tetrameric (four identical subunits) or hetero-tetrameric (a total of four subunits selected from two or more types of subunits). The four subunits are symmetrically arranged around the ion conduction pore. Although the extent of heteromerization has been the subject of some debate, the most recent research in this area suggest that all four thermosensitive TRPVs (1-4) can form heteromers with each other. This result is in line with the general observation that TRP coassembly tends to occur between subunits with high sequence similarities. How TRP subunits recognize and interact with each other is still poorly understood.[6][7]
The TRPV channel monomeric subunit components each contain six transmembrane (TM) domains (designated S1–S6) with a pore domain between the fifth (S5) and sixth (S6) segments.[8] TRPV subunits contain three to five N-terminal ankyrin repeats.[9]
Function
TRPV proteins respond to the taste of garlic (allicin). TRPV1 contributes to heat and inflammation sensations and mediates the pungent odor and pain sensations associated with capsaicin and piperine.
Family members
The table below summarizes the functions and properties of the individual TRPV channel family members:[10][11]
| group | channel | function | tissue distribution | Ca2+/Na+ selectivity |
heteromeric associated subunits | other associated proteins |
|---|---|---|---|---|---|---|
| 1 | TRPV1 | vanilloid (capsaicin) receptor and noxious thermosensor (43 °C) | CNS and PNS | 9:1 | TRPV2, TRPV3 | calmodulin, PI3 kinase |
| TRPV2 | osmo- and noxious heat thermosensor (52 °C) | CNS, spleen and lung | 3:1 | TRPV1 | ||
| TRPV3 | warmth sensor channel (33-39 °C) | Skin, CNS and PNS | 12:1 | TRPV1 | ||
| TRPV4 | osmo- and warmth sensor channel (27-34 °C) | CNS and internal organs;
human sperm[12] |
6:1 | aquaporin 5, calmodulin, pacsin 3 | ||
| 2 | TRPV5 | calcium-selective TRP channel | intestine, kidney, placenta | 100:1 | TRPV6 | annexin II / S100A10, calmodulin |
| TRPV6 | calcium-selective TRP channel | kidney, intestine | 130:1 | TRPV5 | annexin II / S100A10, calmodulin |
Clinical significance
Mutations in TRPs have been linked to neurodegenerative disorders, skeletal dysplasia, kidney disorders,[2] and may play an important role in cancer. TRPs may make important therapeutic targets. There is significant clinical significance to TRPV1, TRPV2, and TRPV3's role as thermoreceptors, and TRPV4's role as mechanoreceptors; reduction of chronic pain may be possible by targeting ion channels involved in thermal, chemical, and mechanical sensation to reduce their sensitivity to stimuli.[13] For instance, the use of TRPV1 agonists would potentially inhibit nociception at TRPV1, particularly in pancreatic tissue where TRPV1 is highly expressed.[14] The TRPV1 agonist capsaicin, found in chili peppers, has been indicated to relieve neuropathic pain.[2] TRPV1 antagonists inhibit nociception at TRPV1.
Role in cancer
Altered expression of TRP proteins often leads to tumorigenesis, clearly seen in TRPM1.[14] Particularly high levels of TRPV6 in prostate cancer have been noted. Such observations could be helpful in following cancer progression and could lead to the development of drugs over activating ion channels, leading to apoptosis and necrosis. Much research remains to be done as to whether TRP channel mutations lead to cancer progression or whether they are associated mutations.
As drug targets
Four TRPVs (TRPV1, TRPV2, TRPV3, and TRPV4) are expressed in afferent nociceptors, pain sensing neurons, where they act as transducers of thermal and chemical stimuli. Agonists, antagonists, or modulators of these channels may find application for the prevention and treatment of pain.[15] A number of TRPV1 selective blockers such as resiniferatoxin are currently in clinical trials for the treatment of various types of pain.[16]
See also
References
- ↑ Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, Rosenmann E, Gonzalez-Nilo F, Latorre R (June 2007). "Dissection of the components for PIP2 activation and thermosensation in TRP channels". Proceedings of the National Academy of Sciences of the United States of America 104 (24): 10246–51. doi:10.1073/pnas.0703420104. PMID 17548815. Bibcode: 2007PNAS..10410246B.
- ↑ 2.0 2.1 2.2 "Linear accelerator as a neurosurgical tool for stereotactic radiosurgery". Neurosurgery 22 (3): 454–64. March 1988. doi:10.1097/00006123-198803000-00002. PMID 3129667.
- ↑ "Abnormal electroretinogram from a Drosophila mutant". Nature 224 (5216): 285–7. October 1969. doi:10.1038/224285a0. PMID 5344615. Bibcode: 1969Natur.224..285C.
- ↑ "Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction". Neuron 2 (4): 1313–23. April 1989. doi:10.1016/0896-6273(89)90069-x. PMID 2516726.
- ↑ Islam MS, ed (January 2011). Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology. 704. Berlin: Springer. pp. 700. ISBN 978-94-007-0264-6.
- ↑ "Vanilloid transient receptor potential cation channels: an overview". Current Pharmaceutical Design 14 (1): 18–31. 2008. doi:10.2174/138161208783330763. PMID 18220815. https://lirias.kuleuven.be/handle/123456789/200319.
- ↑ Cheng W, Yang F, Takanishi CL, Zheng J (March 2007). "Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties". J. Gen. Physiol. 129 (3): 191–207. doi:10.1085/jgp.200709731. PMID 17325193.
- ↑ Vannier B, Zhu X, Brown D, Birnbaumer L (April 1998). "The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry". J. Biol. Chem. 273 (15): 8675–9. doi:10.1074/jbc.273.15.8675. PMID 9535843.
- ↑ Montell C (February 2005). "The TRP superfamily of cation channels". Sci. STKE 2005 (272): re3. doi:10.1126/stke.2722005re3. PMID 15728426.
- ↑ "International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels". Pharmacological Reviews 57 (4): 427–50. December 2005. doi:10.1124/pr.57.4.6. PMID 16382100.
- ↑ "TRP channels". Annual Review of Biochemistry 76 (1): 387–417. 2007. doi:10.1146/annurev.biochem.75.103004.142819. PMID 17579562.
- ↑ "TRPV4 is the temperature-sensitive ion channel of human sperm". eLife 7. July 2018. doi:10.7554/elife.35853. PMID 29963982.
- ↑ "TRP channels: targets for the relief of pain". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1772 (8): 989–1003. August 2007. doi:10.1016/j.bbadis.2007.01.008. PMID 17321113. https://hal.archives-ouvertes.fr/hal-00562760/file/PEER_stage2_10.1016%252Fj.bbadis.2007.01.008.pdf.
- ↑ 14.0 14.1 "TRP channels in cancer". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1772 (8): 937–46. August 2007. doi:10.1016/j.bbadis.2007.05.006. PMID 17616360. https://hal.archives-ouvertes.fr/hal-00562788/file/PEER_stage2_10.1016%252Fj.bbadis.2007.05.006.pdf.
- ↑ Levine JD, Alessandri-Haber N (August 2007). "TRP channels: targets for the relief of pain". Biochim. Biophys. Acta 1772 (8): 989–1003. doi:10.1016/j.bbadis.2007.01.008. PMID 17321113. https://hal.archives-ouvertes.fr/hal-00562760/file/PEER_stage2_10.1016%252Fj.bbadis.2007.01.008.pdf.
- ↑ "The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept". Nature Reviews. Drug Discovery 6 (5): 357–72. May 2007. doi:10.1038/nrd2280. PMID 17464295.
External links
- Transient+Receptor+Potential+Channels at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Transient Receptor Potential Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. http://www.iuphar-db.org/IC/FamilyMenuForward?familyId=12.
- "TRIP Database". a manually curated database of protein-protein interactions for mammalian TRP channels. http://www.trpchannel.org.
 |

