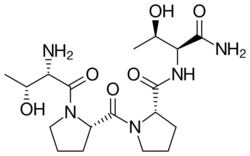
Chemistry:Rapastinel
 | |
 | |
| Clinical data | |
|---|---|
| Other names | GLYX-13; BV-102 |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| Drug class | Selective NMDA receptor modulator |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H31N5O6 |
| Molar mass | 413.475 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rapastinel (INN) (former developmental code name GLYX-13) is a novel antidepressant that was under development by Allergan (previously Naurex) as an adjunctive therapy for the treatment of treatment-resistant depression.[1][2] It is a centrally active, intravenously administered (non-orally active) amidated tetrapeptide that acts as a novel and selective modulator of the NMDA receptor.[1][2][3] The drug is a rapid-acting and long-lasting antidepressant as well as robust cognitive enhancer by virtue of its ability to enhance NMDA receptor-mediated signal transduction and synaptic plasticity.[1][2][3]
Clinical development
On March 3, 2014, the U.S. FDA granted Fast Track designation to the development of rapastinel as an adjunctive therapy in treatment-resistant major depressive disorder.[4] As of 2015, the drug had completed phase II clinical development for this indication and achieved proof of concept as a rapid-acting antidepressant by demonstrating reduced depressive symptoms at days 1 through 7, as assessed by the HAM-D, without eliciting psychotomimetic or other significant side effects.[5] On January 29, 2016, Allergan (who acquired Naurex in July 2015) announced that rapastinel had received Breakthrough Therapy designation from the U.S. FDA for adjunctive treatment of major depressive disorder.[6]
On March 6, 2019, Allergan announced rapastinel failed to differentiate from placebo during phase III trials.[7] Early successful clinical studies of rapastinel in depression spurred the development of next-generation compounds with similar mechanisms of action including apimostinel (GATE-202, NRX-1074), a 2nd generation analog with improved potency, and zelquistinel (GATE-251, AGN-241751), a 3rd generation small molecule with improved potency and high oral bioavailability.[8]
Preclinical development
Rapastinel was originally invented by Joseph Moskal, the co-founder of Naurex, via structural modification of B6B21, a monoclonal antibody that similarly binds to and modulates the NMDA receptor.[2][9][10][11] Rapastinel binds to a novel and unique domain on the NMDA receptor complex that is distinct from the glycine co-agonist binding site.[3][12] Rapastinel exhibits a biphasic dose response in vitro.[3][13] At therapeutically relevant concentrations, rapastinel enhances glutamate-mediated NMDA receptor activity, independent of glycine co-agonism, and enhances the magnitude of NMDAR-mediated synaptic plasticity at excitatory synapses in the mPFC.[3][13] Positive modulation of NMDA receptors by rapastinel produces antidepressant effects that are convergent with the NMDA receptor antagonist ketamine, however, rapastinel has no ketamine-like side effects such as cognitive impairment and psychotomimetic symptoms.[14][15]
In addition to its rapid and sustained antidepressant effects, rapastinel has been shown to enhance memory and learning in both young adult and learning-impaired, aging rat models.[16] It has been shown to increase Schaffer collateral-CA1 long-term potentiation in vitro. In concert with a learning task, rapastinel has also been shown to elevate gene expression of hippocampal NR1, a subunit of the NMDA receptor, in three-month-old rats.[17] Neuroprotective effects have also been demonstrated in Mongolian Gerbils by delaying the death of CA1, CA3, and dentate gyrus pyramidal neurons under glucose and oxygen-deprived conditions.[18]
See also
References
- ↑ 1.0 1.1 1.2 "Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status". CNS Drugs 35 (5): 527–543. May 2021. doi:10.1007/s40263-021-00816-x. PMID 33904154.
- ↑ 2.0 2.1 2.2 2.3 "The Development of Rapastinel (Formerly GLYX-13); A Rapid Acting and Long Lasting Antidepressant". Current Neuropharmacology 15 (1): 47–56. 2016. doi:10.2174/1570159x14666160321122703. PMID 26997507.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology 22 (3): 247–259. March 2019. doi:10.1093/ijnp/pyy101. PMID 30544218.
- ↑ "FDA Grants Fast Track Designation to Naurex's Rapid-Acting Novel Antidepressant GLYX-13" (Press release) – via www.prnewswire.com.
- ↑ "Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent". Journal of Psychiatric Practice 21 (2): 140–149. March 2015. doi:10.1097/01.pra.0000462606.17725.93. PMID 25782764.
- ↑ "Allergan's Rapastinel Receives FDA Breakthrough Therapy Designation for Adjunctive Treatment of Major Depressive Disorder (MDD)". www.prnewswire.com (Press release). Retrieved 2022-05-13.
- ↑ "Allergan Announces Phase 3 Results for Rapastinel as an Adjunctive Treatment of Major Depressive Disorder (MDD)". https://news.abbvie.com/news/allergan-press-releases/allergan-announces-phase-3-results-for-rapastinel-as-an-adjunctive-treatment-major-depressive-disorder-mdd.htm.
- ↑ "Home - Gate Neurosciences" (in en-US). https://www.gateneuro.com/.
- ↑ "Glycine-like modulation of N-methyl-D-aspartate receptors by a monoclonal antibody that enhances long-term potentiation". Journal of Neurochemistry 57 (1): 323–332. July 1991. doi:10.1111/j.1471-4159.1991.tb02131.x. PMID 1828831.
- ↑ "GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator". Neuropharmacology 49 (7): 1077–1087. December 2005. doi:10.1016/j.neuropharm.2005.06.006. PMID 16051282.
- ↑ "The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats". Neurobiology of Aging 32 (4): 698–706. April 2011. doi:10.1016/j.neurobiolaging.2009.04.012. PMID 19446371.
- ↑ "Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons". Neuropsychopharmacology 46 (4): 799–808. March 2021. doi:10.1038/s41386-020-00882-7. PMID 33059355.
- ↑ 13.0 13.1 "A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus". Neuropharmacology 55 (7): 1238–1250. December 2008. doi:10.1016/j.neuropharm.2008.08.018. PMID 18796308.
- ↑ "Cell-type specific modulation of NMDA receptors triggers antidepressant actions". Molecular Psychiatry 26 (9): 5097–5111. September 2021. doi:10.1038/s41380-020-0796-3. PMID 32488125.
- ↑ "Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: Convergent mechanisms". Pharmacology, Biochemistry, and Behavior 188: 172827. January 2020. doi:10.1016/j.pbb.2019.172827. PMID 31733218.
- ↑ "The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats". Neurobiology of Aging 32 (4): 698–706. April 2011. doi:10.1016/j.neurobiolaging.2009.04.012. PMID 19446371.
- ↑ "GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator". Neuropharmacology 49 (7): 1077–1087. December 2005. doi:10.1016/j.neuropharm.2005.06.006. PMID 16051282.
- ↑ "Neuroprotection by a novel NMDAR functional glycine site partial agonist, GLYX-13". NeuroReport 20 (13): 1193–1197. August 2009. doi:10.1097/WNR.0b013e32832f5130. PMID 19623090.
External links
- Rapastinel - AdisInsight
- Rapastinel (GLYX-13) - Naurex, Inc.
- Rapastinel Receives FDA Breakthrough Therapy Designation - Allergan plc. press release
 |

