Chemistry:Reclazepam
From HandWiki
Short description: Drug
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
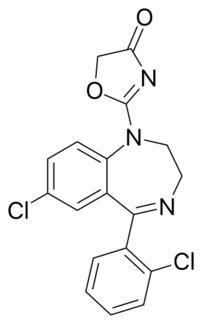
| Formula | C18H13Cl2N3O2 |
| Molar mass | 374.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Reclazepam is a drug which is a benzodiazepine derivative. It has sedative and anxiolytic effects similar to those produced by other benzodiazepine derivatives, and has a short duration of action.[1]
Synthesis

The reduction of the lactam in Delorazepam with lithium aluminium hydride gives CID:20333776 (1). Condensation with 2-chloroacetylisocyanate [4461-30-7] (2) proceeds to afford urea, CID:20333773 (3). Reaction of that with sodium iodide and base probably proceeds initially by halogen exchange of iodine for chlorine (Finkelstein reaction). Subsequent replacement of iodide by the enol anion of the urea oxygen results in formation of the oxazolone ring. There is thus obtained reclazepam (4).
See also
References
- ↑ "Reclazepam". Pscyhotropics.dk. 20 January 1986. http://www.psychotropics.dk/moleculeView/default.aspx?ID=1442&Catalogtype=A&ChapterID=1&Thissortorder=39.[|permanent dead link|dead link}}]
- ↑ Yonan PK, "5-Aryl-1-(2-oxazolin-2-yl)-1H-1,4-benzodiazepines and related compounds", US patent 4208327, issued 17 June 1980, assigned to GD Searle LLC.
- ↑ "α‐Chloroacetyl Isocyanate: Isocyanic acid, anhydride with chloroacetic acid.". Organic Syntheses 46: 16. April 2003. doi:10.1002/0471264180.os046.05.
- ↑ Reeder E, Sternbach LH, "Process for production of benzodiazepines", US patent 3144439, issued 1964, assigned to Hoffmann-La Roche Inc
 |
