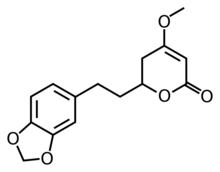
Chemistry:Dihydromethysticin
| |
| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[2-(1,3-benzodioxol-5-yl)ethyl]-4-methoxy-2,3-dihydropyran-6-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | C107882 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H16O5 | |
| Molar mass | 276.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dihydromethysticin is one of the six major kavalactones found in the kava plant.[1]
Pharmacology
Dihydromethysticin has marked activity on the induction of CYP3A23, as does the related chemical desmethoxyyangonin.[2]
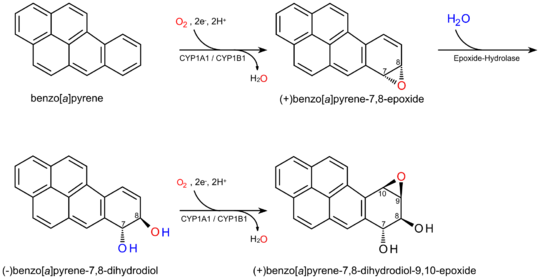
Both dihydromethysticin and methysticin induce the hepatic enzyme CYP1A1, which increases the amount of the very highly carcinogenic benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide in the body (via the metabolism of benzo[a]pyrene) and may be responsible for some of the toxic effects associated with kava consumption.[citation needed]
In vitro, dihydromethysticin possesses analgesic, anticonvulsant, and anxiolytic effects.[3] It has been found to act as a GABAA receptor positive allosteric modulator and as an reversible inhibitor of monoamine oxidase B.[4][5]
References
- ↑ Malani, Joji (2002-12-03). "Evaluation of the effects of Kava on the Liver". Fiji School of Medicine. http://www.spc.int/cis/documents/Kava%20article%20DrMalani.pdf. Retrieved 2009-09-04.
- ↑ Ma, Yuzhong; Karuna Sachdeva; Jirong Liu1; Michael Ford; Dongfang Yang; Ikhlas Khan; Clinton Chichester; Bingfang Yan (November 2004). "Desmethoxyyangonin and dihydromethysticin are two major pharmacological kavalactones with marked activity on the induction of CYP3A23.". Drug Metabolism and Disposition 32 (11): 1317–1324. doi:10.1124/dmd.104.000786. PMID 15282211.
- ↑ "Effects of kawain and dihydromethysticin on field potential changes in the hippocampus.". Progress in Neuro-Psychopharmacology and Biological Psychiatry 21 (4): 697–706. May 1997. doi:10.1016/s0278-5846(97)00042-0. PMID 9194150.
- ↑ Sarris, Jerome; Laporte, Emma; Schweitzer, Isaac (2011). "Kava: A Comprehensive Review of Efficacy, Safety, and Psychopharmacology". Australian & New Zealand Journal of Psychiatry 45 (1): 27–35. doi:10.3109/00048674.2010.522554. PMID 21073405.
- ↑ "Therapeutic potential of kava in the treatment of anxiety disorders". CNS Drugs 16 (11): 731–43. 2002. doi:10.2165/00023210-200216110-00002. PMID 12383029.
External links
 |