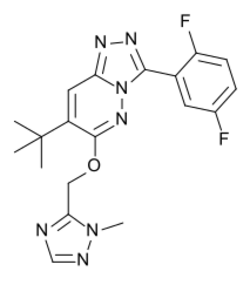
Chemistry:L-838,417
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H19F2N7O |
| Molar mass | 399.406 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme.
L-838,417 is a subtype-selective GABAA positive allosteric modulator, acting as a partial agonist at α2, α3 and α5 subtypes. However, it acts as a negative allosteric modulator at the α1 subtype, and has little affinity for the α4 or α6 subtypes.[1] This gives it selective anxiolytic effects, which are mediated mainly by α2 and α3 subtypes, but with little sedative or amnestic effects as these effects are mediated by α1.[2][3] Some sedation might still be expected due to its activity at the α5 subtype, which can also cause sedation, however no sedative effects were seen in animal studies even at high doses, suggesting that L-838,417 is primarily acting at α2 and α3 subtypes with the α5 subtype of lesser importance.[4][5]
As might be predicted from its binding profile, L-838,417 substitutes for the anxiolytic benzodiazepine chlordiazepoxide in animals, but not for the hypnotic imidazopyridine drug zolpidem.[6][7] The synthesis of L-838,417 and similar compounds was described in 2005 in the Journal of Medicinal Chemistry.[8]
In neuropathic pain animal models, it has been shown that stabilizing the Potassium Chloride Cotranspoter 2 (KCC2) at neuronal membranes could not only potentiate the L-838,417-induced analgesia in rats, but also rescue its analgesic potential at high doses, revealing a novel strategy for analgesia in pathological pain, by combined targeting of the appropriate GABAA receptor subtypes (i.e. α2, α3) and restoring Cl− homeostasis.[9]
See also
References
- ↑ "Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype". Nature Neuroscience 3 (6): 587–92. June 2000. doi:10.1038/75761. PMID 10816315.
- ↑ "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opinion on Investigational Drugs 14 (5): 601–18. May 2005. doi:10.1517/13543784.14.5.601. PMID 15926867.
- ↑ "Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm". The European Journal of Neuroscience 23 (9): 2495–504. May 2006. doi:10.1111/j.1460-9568.2006.04775.x. PMID 16706856.
- ↑ "Comparative effects of nonselective and subtype-selective gamma-aminobutyric acidA receptor positive modulators in the rat-conditioned emotional response test". Behavioural Pharmacology 18 (3): 191–203. May 2007. doi:10.1097/FBP.0b013e32814fcdd4. PMID 17426483.
- ↑ "Pharmacological and computational analysis of alpha-subunit preferential GABA(A) positive allosteric modulators on the rat septo-hippocampal activity". Neuropharmacology 52 (3): 733–43. March 2007. doi:10.1016/j.neuropharm.2006.09.022. PMID 17113111.
- ↑ "Comparative cue generalization profiles of L-838, 417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination". The Journal of Pharmacology and Experimental Therapeutics 316 (3): 1291–9. March 2006. doi:10.1124/jpet.105.094003. PMID 16339395.
- ↑ "A comparison of chlordiazepoxide, bretazenil, L838,417 and zolpidem in a validated mouse Vogel conflict test". Psychopharmacology 182 (4): 475–84. November 2005. doi:10.1007/s00213-005-0119-z. PMID 16133136.
- ↑ "7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine: a functionally selective gamma-aminobutyric acid(A) (GABA(A)) alpha2/alpha3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models". Journal of Medicinal Chemistry 48 (23): 7089–92. November 2005. doi:10.1021/jm058034a. PMID 16279764.
- ↑ "A-mediated analgesia in neuropathic pain". Nature Communications 11 (1): 869. February 2020. doi:10.1038/s41467-019-14154-6. PMID 32054836. Bibcode: 2020NatCo..11..869L.
 |
