Chemistry:Carisbamate
 | |
| Clinical data | |
|---|---|
| Trade names | Comfyde (proposed) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
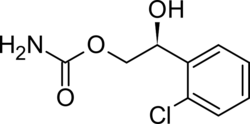
| Formula | C9H10ClNO3 |
| Molar mass | 215.63 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Carisbamate (YKP 509, proposed trade name Comfyde) is an experimental anticonvulsant drug that was under development by Johnson & Johnson Pharmaceutical Research and Development but never marketed.
Clinical study
A phase II clinical trial in the treatment of partial seizures demonstrated that the compound has efficacy in the treatment of partial seizures and a good safety profile. Since late 2006, the compound has been undergoing a large multicenter phase III clinical trial for the treatment of partial seizures. Its mechanism of action is unknown.[1][2]
A double-blind, placebo-controlled trial of carisbamate in 323 patients with migraine determined that carisbamate was well tolerated at doses up to 600 mg/day, but it failed to demonstrate that the drug was sufficiently more effective than placebo in migraine prophylaxis.[3]
History
In 1998, the compound was in-licensed from SK Corp. (currently Life Science Business Division of SK Holdings), a South Korean company. On October 24, 2008, Johnson & Johnson announced that it had submitted a New Drug Application to the U.S. Food and Drug Administration (FDA) for carisbamate.[4] Johnson & Johnson received provisional approval by the FDA to market carisbamate under the brand name of Comfyde. However, on August 21, 2009, Johnson & Johnson reported that the FDA had failed to give marketing approval.
References
- ↑ Rogawski MA (2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Res 69 (3): 273–294. doi:10.1016/j.eplepsyres.2006.02.004. PMID 16621450.
- ↑ "Carisbamate (RWJ-333369)". Neurotherapeutics 4 (1): 106–109. 2007. doi:10.1016/j.nurt.2006.11.016. PMID 17199023.
- ↑ "Evaluation of carisbamate for the treatment of migraine in a randomized, double-blind trial". Headache 49 (2): 216–226. 2009. doi:10.1111/j.1526-4610.2008.01326.x. PMID 19222595.
- ↑ "Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Submits New Drug Application to FDA for Carisbamate" (Press release). Johnson & Johnson. 2008-10-24. Retrieved 2008-11-02.
 |
