Chemistry:Nifoxipam
From HandWiki
Short description: Benzodiazepine designer drug
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
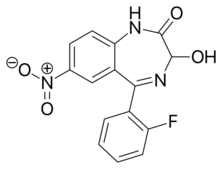
| Formula | C15H10FN3O4 |
| Molar mass | 315.260 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nifoxipam (3-hydroxydesmethylflunitrazepam, DP 370) is a benzodiazepine that is a minor metabolite of flunitrazepam and has been sold online as a designer drug.[1][2][3][4][5][6][7][8][9]
Nifoxipam produces strong tranquillising and sleep-prolonging effects and has much lower toxicity compared to lormetazepam and flunitrazepam in mice.[1]
See also
References
- ↑ 1.0 1.1 Posselt K, Wagener HH, Gruber K,, "Pharmaceutical composition containing 5-(2-fluorophenyl)-1,3-dihydro-3-hydroxy-7-nitro- or 5-(2-fluorophenyl)-1,3-dihydro-3-hydroxy-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one and process for their preparation", EP patent 0158267, published 16 October 1985, assigned to Dolorgiet Beteiligungs-GmbH
- ↑ "Nifoxipam". New Synthetic Drugs Database. http://nsddb.eu/substance/478/.
- ↑ "Flunitrazepam metabolism by cytochrome P450S 2C19 and 3A4". Drug Metabolism and Disposition 29 (4 Pt 1): 460–5. April 2001. PMID 11259331. http://dmd.aspetjournals.org/content/29/4/460.long.
- ↑ "Designer benzodiazepines: A new challenge". World Psychiatry 14 (2): 248. June 2015. doi:10.1002/wps.20236. PMID 26043347.
- ↑ Kevin Flemen (August 2015). "Drug Facts - Newer Unregulated Drugs". KFx. http://www.kfx.org.uk/drug_facts/drug_facts_images_and_pdfs/researchchemicals_4.2015.pdf.
- ↑ "Nifoxipam". WEDINOS. http://www.wedinos.org/db/materials/view/00713.
- ↑ "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry 408 (13): 3571–91. May 2016. doi:10.1007/s00216-016-9439-6. PMID 27071765.
- ↑ "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis 9 (4): 640–645. April 2017. doi:10.1002/dta.2003. PMID 27366870.
- ↑ "Metabolites replace the parent drug in the drug arena. The cases of fonazepam and nifoxipam". Forensic Toxicology 35 (1): 1–10. 2016. doi:10.1007/s11419-016-0338-5. PMID 28127407.
 |

