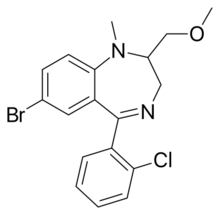
Chemistry:Metaclazepam
 | |
| Clinical data | |
|---|---|
| Trade names | Talis |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H18BrClN2O |
| Molar mass | 393.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Metaclazepam[1] (marketed under the brand name Talis) is a drug which is a benzodiazepine derivative.[2][3] It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam.[4] It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam.[5] There is no significant difference in metabolism between younger and older individuals.[6]
Metaclazepam is slightly more effective as an anxiolytic than bromazepam,[7] or diazepam,[8] with a 15 mg dose of metaclazepam equivalent to 4 mg of bromazepam.[9] Metaclazepam can interact with alcohol producing additive sedative-hypnotic effects.[6][10] Fatigue is a common side effect from metaclazepam at high doses.[11] Small amounts of metaclazepam as well as its metabolites enter into human breast milk.[12]
See also
References
- ↑ US patent 4098786
- ↑ "Metabolism and pharmacokinetics of metaclazepam (Talis), Part III: Determination of the chemical structure of metabolites in dogs, rabbits and men". European Journal of Drug Metabolism and Pharmacokinetics 9 (4): 325–46. 1984. doi:10.1007/bf03189684. PMID 6532806.
- ↑ "Analytical profile of metaclazepam". Arzneimittel-Forschung 36 (9): 1302–6. September 1986. PMID 3790179.
- ↑ "General pharmacology of the anxiolytic compound metaclazepam in comparison to other benzodiazepines". Arzneimittel-Forschung 35 (11): 1643–55. 1985. PMID 2868732.
- ↑ "Pharmacokinetic profile of metaclazepam (Talis), a new 1.4-benzodiazepine. Influence of different dosage regimens on the pharmacokinetic profile of metaclazepam and its main metabolite under steady-state conditions". European Journal of Drug Metabolism and Pharmacokinetics 11 (3): 205–10. 1986. doi:10.1007/bf03189848. PMID 3816876.
- ↑ 6.0 6.1 "Comparison of the pharmacokinetic profile of metaclazepam in old and young volunteers". European Journal of Clinical Pharmacology 29 (2): 247–9. 1985. doi:10.1007/bf00547431. PMID 4076323.
- ↑ "A double-blind comparison of the anxiolytic activity of two benzodiazepines, metaclazepam and bromazepam, in anxiety neurosis". Current Medical Research and Opinion 11 (1): 45–7. 1988. doi:10.1185/03007998809111130. PMID 2898321. https://zenodo.org/record/1236200.
- ↑ "Double-blind study of metaclazepam versus diazepam treatment of outpatients with anxiety syndrome". Pharmacopsychiatry 22 (3): 120–5. May 1989. doi:10.1055/s-2007-1014593. PMID 2568645.
- ↑ "Controlled study on the anxiolytic activity of a newly-developed benzodiazepine, metaclazepam". Current Medical Research and Opinion 11 (1): 41–4. 1988. doi:10.1185/03007998809111129. PMID 2898320.
- ↑ "[Experimental studies on the interaction of alcohol and metaclazepam]". Beiträge zur Gerichtlichen Medizin 41: 413–7. 1983. PMID 6639614.
- ↑ "Double-blind randomized trial of the benzodiazepine derivative metaclazepam as compared with placebo treatment of outpatients with anxiety syndromes". Pharmacopsychiatry 21 (3): 136–43. May 1988. doi:10.1055/s-2007-1014665. PMID 2900514.
- ↑ "Transfer of metaclazepam and its metabolites into breast milk". Arzneimittel-Forschung 39 (11): 1468–70. November 1989. PMID 2575907.
 |
