Chemistry:Fluroxene
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
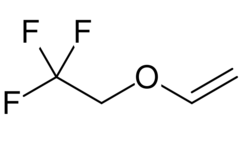
| Formula | C4H5F3O |
| Molar mass | 126.078 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fluroxene (INN, USAN; brand name Fluoromar), or 2,2,2-trifluoroethyl vinyl ether, is a volatile, inhalational anesthetic.[1][2] It was synthesized in 1951, and was introduced for clinical use in 1954, but was voluntarily withdrawn from the market in 1974 due to its potential flammability and accumulating evidence that it could cause organ toxicity.[2][1][3] In any case, prior to being discontinued, it had largely been superseded by halothane.[4] Fluroxene is metabolized to 2,2,2-trifluoroethanol, a compound responsible for some of the toxicity seen with fluroxene use.[5][6]
See also
References
- ↑ 1.0 1.1 "Inhaled Anesthetics". Pharmacology and Physiology in Anesthetic Practice. Lippincott Williams & Wilkins. 11 January 2012. pp. 142–. ISBN 978-1-4511-6583-8. https://books.google.com/books?id=lHhGtkvOoM0C&pg=PT142.
- ↑ 2.0 2.1 "Chapter 1: the History of Anesthesia". Clinical Anesthesia. Lippincott Williams & Wilkins. 1 January 2011. pp. 113–. ISBN 978-1-4511-2297-8. https://books.google.com/books?id=vGtSChnRRJ8C&pg=PT113.
- ↑ Introduction to the practice of anesthesia. Medical Dept., Harper & Row. 1 January 1978. ISBN 978-0-06-141534-0. https://books.google.com/books?id=ecZpAAAAMAAJ.
- ↑ Acta anaesthesiologica Belgica. Acta Medica Belgica. 1974. https://books.google.com/books?id=KmsxAAAAIAAJ.
- ↑ "Metabolism and toxicity of 2,2,2-trifluoroethyl vinyl ether". Environmental Health Perspectives 21: 225–230. December 1977. doi:10.1289/ehp.7721225. PMID 25763.
- ↑ "Multiple aspects of the toxicity of fluroxene and its metabolite 2,2,2-trifluoroethanol". Critical Reviews in Toxicology 19 (2): 87–112. 1988. doi:10.3109/10408448809014901. PMID 2906849.
 |
