Chemistry:Zuranolone
 | |
| Clinical data | |
|---|---|
| Pronunciation | /zʊˈrænəloʊn/ zuu-RAN-ə-lohn |
| Trade names | Zurzuvae |
| Other names | SAGE-217; S-812217; SGE-797; BIIB-125 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Neurosteroid; GABAA receptor positive allosteric modulator |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.5%[3][unreliable medical source?] |
| Metabolism | CYP3A4[3][unreliable medical source?] |
| Elimination half-life | 16–23 hours[4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
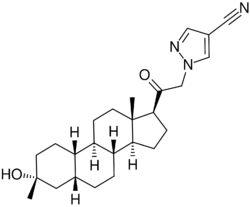
| Formula | C25H35N3O2 |
| Molar mass | 409.574 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zuranolone, sold under the brand name Zurzuvae, is a medication used for the treatment of postpartum depression.[1][2] It is taken by mouth.[1]
The most common side effects include drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection.[1][2] An orally active inhibitory pregnane neurosteroid, zuranolone acts as a positive allosteric modulator of the GABAA receptor.[6][7][8]
Zuranolone was approved for medical use in the United States for the treatment of postpartum depression in August 2023.[2] It was developed by Sage Therapeutics and Biogen.[9]
Medical uses
Zuranolone is indicated for the treatment of postpartum depression.[1][2]
Adverse effects
The most common side effects include drowsiness, dizziness, diarrhea, fatigue, and urinary tract infection.[2]
The US FDA label contains a boxed warning noting that zuranolone can impact a person's ability to drive and perform other potentially hazardous activities.[2] Use of zuranolone may cause suicidal thoughts and behavior.[2] Zuranolone may cause fetal harm.[2]
History
Zuranolone was developed as an improvement on the intravenously administered neurosteroid brexanolone, with high oral bioavailability and a biological half-life suitable for once-daily administration.[7][10] Its half-life is around 16 to 23 hours, compared to approximately 9 hours for brexanolone.[4][5]
The efficacy of zuranolone for the treatment of postpartum depression in adults was demonstrated in two randomized, double-blind, placebo-controlled, multicenter studies.[2] The trial participants were women with postpartum depression who met the Diagnostic and Statistical Manual of Mental Disorders criteria for a major depressive episode and whose symptoms began in the third trimester or within four weeks of delivery.[2] In study 1, participants received 50 mg of zuranolone or placebo once daily in the evening for 14 days.[2] In study 2, participants received another zuranolone product that was approximately equal to 40 mg of zuranolone or placebo, also for 14 days.[2] Participants in both studies were monitored for at least four weeks after the 14-day treatment.[2] The primary endpoint of both studies was the change in depressive symptoms using the total score from the 17-item Hamilton depression rating scale (HAMD-17), measured at day 15.[2] Participants in the zuranolone groups showed significantly more improvement in their symptoms compared to those in the placebo groups.[2] The treatment effect was maintained at day 42—four weeks after the last dose of zuranolone.[2]
Society and culture
Zuranolone is the international nonproprietary name.[11]
Legal status
Zuranolone was approved by the US Food and Drug Administration (FDA) for the treatment of postpartum depression in August 2023.[2][12] The FDA granted the application for zuranolone priority review and fast track designations.[2] Approval of Zurzuvae was granted to Sage Therapeutics, Inc.[2]
Zuranolone has also been under development for the treatment of major depressive disorder, but the application for this use was given a Complete Response Letter (CRL) by the FDA due to insufficient evidence of effectiveness.[13]
Research
In a randomized, placebo-controlled phase III trial to assess its efficacy and safety for the treatment of major depressive disorder, subjects in the zuranolone group (50 mg oral zuranolone once daily for 14 days) experienced statistically significant and sustained improvements in depressive symptoms (as measured by HAM-D score) throughout the treatment and follow-up periods of the study.[14]
Other investigational applications include insomnia, bipolar depression, essential tremor, and Parkinson's disease.[15][6][16]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Zurzuvae (zuranolone) capsules, for oral use, [controlled substance schedule pending"]. https://documents.sage-biogen.com/us/zurzuvae/pi.pdf.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 "FDA Approves First Oral Treatment for Postpartum Depression". U.S. Food and Drug Administration (FDA) (Press release). 4 August 2023. Retrieved 4 August 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 "Zuranolone". https://go.drugbank.com/drugs/DB15490.
- ↑ 4.0 4.1 "GABAkines - Advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors". Pharmacology & Therapeutics 234: 108035. 2022. doi:10.1016/j.pharmthera.2021.108035. PMID 34793859.
- ↑ 5.0 5.1 "Intravenous brexanolone for postpartum depression: what it is, how well does it work, and will it be used?". Therapeutic Advances in Psychopharmacology 10: 2045125320968658. 2020. doi:10.1177/2045125320968658. PMID 33224470.
- ↑ 6.0 6.1 "SAGE 217". http://adisinsight.springer.com/drugs/800043382.
- ↑ 7.0 7.1 "Breakthroughs in neuroactive steroid drug discovery". Bioorganic & Medicinal Chemistry Letters 28 (2): 61–70. 2018. doi:10.1016/j.bmcl.2017.11.043. PMID 29223589.
- ↑ "Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1'-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (γ-Aminobutyric Acid)A Receptor". Journal of Medicinal Chemistry 60 (18): 7810–7819. 2017. doi:10.1021/acs.jmedchem.7b00846. PMID 28753313.
- ↑ Saltzman, Jonathan (4 August 2023). "FDA approves postpartum depression pill from two Cambridge drug firms". The Boston Globe. https://www.bostonglobe.com/2023/08/04/business/fda-postpartum-depression-pill/.
- ↑ "Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator". Neuropharmacology 181: 108333. 2020. doi:10.1016/j.neuropharm.2020.108333. PMID 32976892.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information 33 (3). 2019.
- ↑ "FDA Approves Zurzuvae (zuranolone), the First and Only Oral Treatment Approved for Women with Postpartum Depression, and Issues a Complete Response Letter for Major Depressive Disorder" (Press release). Biogen Inc. 4 August 2023. Retrieved 4 August 2023 – via GlobeNewswire.
- ↑ McKenzie, Heather. "Sage Hints at Difficult Decisions After Zuranolone's Rejection in MDD". https://www.biospace.com/article/sage-hints-at-difficult-decisions-after-zuranolone-s-rejection-in-mdd-/.
- ↑ "Zuranolone for the Treatment of Adults With Major Depressive Disorder: A Randomized, Placebo-Controlled Phase 3 Trial". The American Journal of Psychiatry 180 (9): 676–684. May 2023. doi:10.1176/appi.ajp.20220459. PMID 37132201.
- ↑ "Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial". JAMA Psychiatry 78 (9): 951–959. 2021. doi:10.1001/jamapsychiatry.2021.1559. PMID 34190962.
- ↑ "Zuranolone as an oral adjunct to treatment of Parkinsonian tremor: A phase 2, open-label study". Journal of the Neurological Sciences 421: 117277. 2021. doi:10.1016/j.jns.2020.117277. PMID 33387701.
External links
- Clinical trial number NCT04442503 for "A Study to Evaluate the Efficacy and Safety of SAGE-217 in Participants With Severe Postpartum Depression (PPD)" at ClinicalTrials.gov
- Clinical trial number NCT02978326 for "A Study to Evaluate SAGE-217 in Participants With Severe Postpartum Depression" at ClinicalTrials.gov
 |

