Chemistry:3-Hydroxyphenazepam
From HandWiki
Short description: Benzodiazepine medication
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
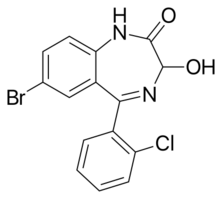
| Formula | C15H10BrClN2O2 |
| Molar mass | 365.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
3-Hydroxyphenazepam is a benzodiazepine with hypnotic, sedative, anxiolytic, and anticonvulsant properties.[1] It is an active metabolite of phenazepam,[1][2] as well as the active metabolite of the benzodiazepine prodrug cinazepam.[3] Relative to phenazepam, 3-hydroxyphenazepam has diminished myorelaxant properties, but is about equivalent in most other regards.[1] Like other benzodiazepines, 3-hydroxyphenazepam behaves as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor with an EC50 value of 10.3 nM.[4][5][6] It has been sold online as a designer drug.[7][8][9][10]
See also
- Lorazepam, licensed medication
- Nifoxipam
- Nitemazepam
References
- ↑ 1.0 1.1 1.2 Drug dependence and emotional behavior: neurophysiological and neurochemical approaches. Consultants Bureau. 31 May 1986. ISBN 978-0-306-10984-3. https://books.google.com/books?id=GiNtAAAAMAAJ.
- ↑ Thin Layer Chromatography in Drug Analysis. CRC Press. 20 December 2013. pp. 299–. ISBN 978-1-4665-0715-9. https://books.google.com/books?id=LSEtAgAAQBAJ&pg=PA299.
- ↑ "Elimination kinetics of the novel prodrug cinazepam possessing psychotropic activity in mice". Pharmacological Reports 63 (5): 1093–1100. 2011. doi:10.1016/s1734-1140(11)70628-4. PMID 22180351.
- ↑ "Phenazepam Pre-Review Report". World Health Organization (WHO). November 2015. https://www.who.int/medicines/access/controlled-substances/5.8_Phenazepam_PreRev.pdf.
- ↑ "Modulation of GABA-activated currents by phenazepam and its metabolites in isolated rat purkinje neurons". Neurophysiology 32 (3): 192. 2000. doi:10.1007/BF02506568. ISSN 0090-2977.
- ↑ "Pharmacodynamical and Neuroreceptor Analysis of the Permeability of the Blood-Brain Barrier for Derivatives of 1,4-Benzodiazepine". Neurophysiology 46 (3): 199–205. 2014. doi:10.1007/s11062-014-9429-2. ISSN 0090-2977.
- ↑ "3-hydroxyphenazepam". New Synthetic Drugs Database. http://nsddb.eu/substance/589/.
- ↑ "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis 9 (4): 640–645. April 2017. doi:10.1002/dta.2003. PMID 27366870.
- ↑ "Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines - an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam and nitrazolam". Journal of Mass Spectrometry 51 (11): 1080–1089. November 2016. doi:10.1002/jms.3840. PMID 27535017. Bibcode: 2016JMSp...51.1080M.
- ↑ "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis 10 (8): 1258–1269. March 2018. doi:10.1002/dta.2387. PMID 29582576. https://rke.abertay.ac.uk/en/publications/527a634d-decc-4d3a-bdca-08659bb13ed6.
 |

