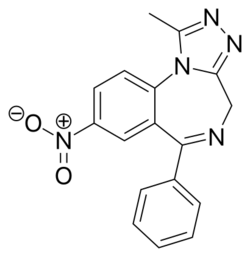
Chemistry:Nitrazolam
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H13N5O2 |
| Molar mass | 319.324 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nitrazolam is a triazolobenzodiazepine (TBZD) , which are benzodiazepine (BZD) derivatives,[1] that has been sold online as a designer drug.[2][3]
It is closely related to clonazolam or flunitrazolam, only differing by the removal of a chlorine or fluorine group respectively at the benzene ring.
A study in mice indicated that nitrazolam can be several times more potent than diazepam as an antagonist of electroshock-induced tonic-extensor convulsions but less potent than diazepam at preventing the righting reflex.[4]
Nitrazolam has been used as an example compound to demonstrate the microscale synthesis of reference materials utilizing polymer‐supported reagents.[5]
Legal status
United Kingdom
In the UK, nitrazolam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[6]
See also
- Adinazolam
- Alprazolam (licensed)
- Flubromazolam
- Nifoxipam
- Nitemazepam
- Pyrazolam
- Triazolam (licensed)
References
- ↑ "Patent US3987052 - 6-Phenyl-4H-s-triazolo[4,3-a[1,4]benzodiazepines"]. The Upjohn Company. 19 October 1976. http://www.google.com/patents/US3987052.
- ↑ "Nitrazolam". New Synthetic Drugs Database. http://nsddb.eu/substance/574/.
- ↑ "Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines - an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam and nitrazolam". Journal of Mass Spectrometry 51 (11): 1080–1089. November 2016. doi:10.1002/jms.3840. PMID 27535017. Bibcode: 2016JMSp...51.1080M.
- ↑ "6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity". Journal of Medicinal Chemistry 14 (11): 1078–81. November 1971. doi:10.1021/jm00293a015. PMID 5165540.
- ↑ "An approach to shortening the timeframe between the emergence of new compounds on the drugs market and the availability of reference standards: The microscale syntheses of nitrazolam and clonazolam for use as reference materials, utilizing polymer-supported reagents". Drug Testing and Analysis 10 (7): 1198–1208. March 2018. doi:10.1002/dta.2383. PMID 29542872. http://researchonline.ljmu.ac.uk/id/eprint/8261/13/An%20approach%20to%20shortening%20the%20timeframe%20between%20the%20emergence%20of%20new%20compounds%20on%20the%20drugs%20market%20and%20the%20availability%20of%20reference%20standards.pdf.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2017". http://www.legislation.gov.uk/uksi/2017/634/contents/made.
 |
