Chemistry:3-Methylbutanoic acid

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylbutanoic acid | |
| Other names
Delphinic acid
3-Methylbutyric acid Isopentanoic acid Isovaleric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H10O2 | |
| Molar mass | 102.13 g/mol |
| Density | 0.925 g/cm3 |
| Melting point | −29 °C (−20 °F; 244 K) |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
| log P | 1.16 |
| Acidity (pKa) | 4.8 (H2O) |
| -67.7·10−6 cm3/mol | |
| Related compounds | |
Related carboxylic acids
|
butyric acid β-hydroxybutyric acid β-hydroxy β-methylbutyric acid |
Related compounds
|
Valeric acid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P280, P301+330+331, P303+361+353, P304+340+310, P305+351+338, P363 | |
| Flash point | 80 °C (176 °F; 353 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.
History
3-Methylbutanoic acid is a minor constituent of the perennial flowering plant valerian (Valeriana officinalis), from which it got its trivial name isovaleric acid: an isomer of valeric acid which shares its unpleasant odor.[2] The dried root of this plant has been used medicinally since antiquity.[3][4] Their chemical identity was first investigated in the 19th century by oxidation of the components of fusel alcohol, which includes the five-carbon amyl alcohols.[5]
Manufacture
In industry, 3-methylbutanoic acid is produced by the hydroformylation[6] of isobutylene with syngas, forming isovaleraldehyde,[7] which is oxidised to the final product.[8]
- (CH3)2C=CH2 + H2 + CO → (CH3)2CHCH2CHO → 3-methylbutanoic acid
Reactions
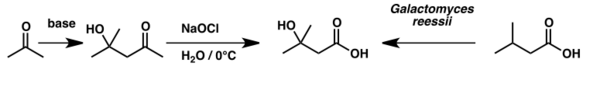
3-Methylbutanoic acid reacts as a typical carboxylic acid: it can form amide, ester, anhydride, and chloride derivatives.[9] The acid chloride is commonly used as the intermediate to obtain the others. The acid has been used to synthesize β-hydroxyisovaleric acid – otherwise known as β-hydroxy β-methylbutyric acid – via microbial oxidation by the fungus Galactomyces reessii.[10]
|
Uses
Isovaleric acid has a strong pungent cheesy or sweaty smell,[11] but its volatile esters such as ethyl isovalerate[12] have pleasant odors and are widely used in perfumery. It is also the primary flavor added to wine when made using Brettanomyces yeasts.[13] Other compounds produced by Brettanomyces yeasts include 4-ethylphenol, 4-vinylphenol, and 4-ethylguaiacol.[14] An excess of isovaleric acid in wine is generally seen as a defect,[14] as it can smell sweaty, leathery, or "like a barnyard", but in small amounts it can smell smokey, spicy, or medicinal.[13] These phenomena may be prevented by killing any Brettanomyces yeasts, such as by sterile filtration, by the addition of relatively large quantities of sulfur dioxide and sometimes sorbic acid, by mixing in alcoholic spirit to give a fortified wine of sufficient strength to kill all yeast and bacteria, or by pasteurization. Isovaleric acid can also be found in beer, and, excepting some English–style ales, is usually considered a flaw.[15] It can be produced by the oxidation of hop resins, or by Brettanomyces yeasts present.[15]
The compound's safety as a food additive was reviewed by an FAO and WHO panel, who concluded that there were no concerns at the likely levels of intake.[16]
Biology
Since isovaleric acid and its esters are natural components of many foods, it is present in mammals including humans.[17] Also, Isovaleryl-coenzyme A is an intermediate in the metabolism of branched-chain amino acids.[18]
Isovaleric acid is a major component of the cause of intense foot odor, as it is produced by skin bacteria metabolizing leucine and in rare cases a condition called isovaleric acidemia can lead to heightened levels of this metabolite.[19]
Salts and esters
An isovalerate or 3-methylbutanoate ion is (CH3)2CHCH2COO−, the conjugate base of the acid. It is the form found in biological systems at physiological pH. An isovalerate or 3-methylbutanoate compound is a salt or ester of the acid.
Examples
See also
- 2-Methylbutanoic acid, an isomer
- Valeric acid, an isomer
References
- ↑ Sigma-Aldrich. "Isovaleric acid". https://www.sigmaaldrich.com/catalog/product/aldrich/129542?lang=en®ion=GB.
- ↑ Chisholm, Hugh, ed (1911). "Valeric Acid". Encyclopædia Britannica. 27 (11th ed.). Cambridge University Press. p. 859.
- ↑ Patočka, Jiří; Jakl, Jiří (2010). "Biomedically relevant chemical constituents of Valeriana officinalis". Journal of Applied Biomedicine 8: 11–18. doi:10.2478/v10136-009-0002-z.
- ↑ Eadie, Mervyn J. (November 2004). "Could Valerian Have Been the First Anticonvulsant?". Epilepsia 45 (11): 1338–1343. doi:10.1111/j.0013-9580.2004.27904.x. PMID 15509234.
- ↑ Pedler, Alexander (1868). "On the isomeric forms of valeric acid". Journal of the Chemical Society 21: 74–76. doi:10.1039/JS8682100074. https://zenodo.org/record/2175638.
- ↑ Franke, Robert; Selent, Detlef; Börner, Armin (2012). "Applied Hydroformylation". Chemical Reviews 112 (11): 5675–5732. doi:10.1021/cr3001803. PMID 22937803.
- ↑ Kohlpaintner, Christian; Schulte, Markus; Falbe, Jürgen; Lappe, Peter; Weber, Jürgen; Frey, Guido D. (2013). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_321.pub3. ISBN 978-3527306732.
- ↑ Riemenschneider, Wilhelm (2000). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a05_235. ISBN 3527306730.
- ↑ Jenkins, P. R. (1985). "Carboxylic acids and derivatives". General and Synthetic Methods. 7. pp. 96–160. doi:10.1039/9781847556196-00096. ISBN 978-0-85186-884-4.
- ↑ "Conversion of beta-methylbutyric acid to beta-hydroxy-beta-methylbutyric acid by Galactomyces reessii". Applied and Environmental Microbiology 63 (11): 4191–5. 1997. doi:10.1128/AEM.63.11.4191-4195.1997. PMID 9361403. Bibcode: 1997ApEnM..63.4191L.
- ↑ "Isovaleric acid". http://www.thegoodscentscompany.com/data/rw1056411.html.
- ↑ "Ethyl 3-methylbutanoate". http://www.thegoodscentscompany.com/data/rw1007041.html.
- ↑ Jump up to: 13.0 13.1 Jackson, Ron S. (2008). Wine Science: Principles and Applications (3rd ed.). Academic Press. p. 495. ISBN 9780123736468. https://books.google.com/books?id=lU4HO2FeWoEC&pg=PA495.
- ↑ Jump up to: 14.0 14.1 Kirk-Othmer (2007). "Wine". Food and Feed Technology, Volume 2. John Wiley & Sons. p. 702. ISBN 9780470174487. https://books.google.com/books?id=f--1V1ftgtsC&pg=RA1-PA705.
- ↑ Jump up to: 15.0 15.1 Oliver, Garrett, ed (2012). The Oxford Companion to Beer. Oxford University Press. p. 498. ISBN 9780195367133. https://books.google.com/books?id=Ga4MYyZq-RMC&pg=PA498.
- ↑ FAO/WHO Expert Committee on food additives (1998). "Safety evaluation of certain food additives and contaminants". http://www.inchem.org/documents/jecfa/jecmono/v040je11.htm.
- ↑ "Metabocard for Isovaleric acid". 2020-02-26. https://hmdb.ca/metabolites/HMDB0000718.
- ↑ Wilson, Jacob M.; Fitschen, Peter J.; Campbell, Bill; Wilson, Gabriel J.; Zanchi, Nelo; Taylor, Lem; Wilborn, Colin; Kalman, Douglas S. et al. (2013). "International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition 10 (1): 6. doi:10.1186/1550-2783-10-6. PMID 23374455.
- ↑ Ara, Katsutoshi; Hama, Masakatsu; Akiba, Syunichi; Koike, Kenzo; Okisaka, Koichi; Hagura, Toyoki; Kamiya, Tetsuro; Tomita, Fusao (April 2006). "Foot odor due to microbial metabolism and its control". Canadian Journal of Microbiology 52 (4): 357–364. doi:10.1139/w05-130. PMID 16699586.
 |