Chemistry:Despropionyl-p-fluorofentanyl
From HandWiki
Short description: Synthetic opioid analgesic precursor
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
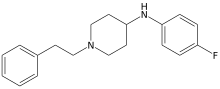
| Formula | C19H23FN2O0 |
| Molar mass | 298.405 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Despropionyl-p-fluorofentanyl is an inactive synthetic opioid analgesic drug precursor to 4-fluorofentanyl. It is an analog of fentanyl.[1][2]
See also
- 3-Methylbutyrfentanyl
- 4-Fluorobutyrfentanyl
- 4-Fluorofentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Benzylfentanyl
- Furanylfentanyl
- Homofentanyl
- List of fentanyl analogues
References
- ↑ Wharton, Rebekah E (2021). "Detection of 30 fentanyl analogs by commercial immunoassay kits". Journal of Analytical Toxicology 45 (2): 111–116. doi:10.1093/jat/bkaa181. PMID 33580693.
- ↑ Carroll, F. Ivy (2021). "Designer drugs: A medicinal chemistry perspectiveq". Annals of the New York Academy of Sciences 1489 (1): 48–77. doi:10.1111/nyas.14349. PMID 32396701.
Further reading
- "Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues". Forensic Toxicology 26 (1): 1–5. June 2008. doi:10.1007/s11419-007-0039-1.
- "Fentanyl receptor assay. II. Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine". J Anal Toxicol 16 (1): 36–41. 1992. doi:10.1093/jat/16.1.36. PMID 1322477.
- "Evaluation of new compounds for opioid activity: 1987 annual report". NIDA Res. Monogr. 81: 543–90. 1988. PMID 3136388.
- "Dependence studies of new compounds in the rhesus monkey, rat, and mouse, 1987". NIDA Res. Monogr. 81: 485–542. 1988. PMID 3136386.
- "Carbon-13 nuclear magnetic resonance spectra of fentanyl analogs". Journal of Heterocyclic Chemistry 26 (3): 677–686. 2009. doi:10.1002/jhet.5570260329.
 |

