Chemistry:Pregnenolone (medication)
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arthenolone, Bina-Skin, Enelone, Natolone, Pregneton, Prenolone, Regnosone, Sharmone, Skinostelon[1][2] |
| Other names | P5; 5-Pregnenolone; δ5-Pregnene-3β-ol-20-one; Pregn-5-en-3β-ol-20-one; NSC-1616 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, transdermal |
| Drug class | Neurosteroid; Anti-inflammatory |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.485 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pregnenolone, sold under the brand name Enelone among others, is a medication and supplement as well as a naturally occurring and endogenous steroid.[3][1][4][5][6] It is described as a neurosteroid and anti-inflammatory drug and was used in the treatment of rheumatoid arthritis and soft-tissue rheumatism in the 1950s but is no longer used today.[3][2][4] Pregnenolone can be taken by mouth, as a topical medication, or by injection into muscle.[3][2]
Pregnenolone is promoted online with false claims that it can treat a variety of health conditions including cancer, arthritis and multiple sclerosis.[7]
Medical uses
Pregnenolone was approved for use as a pharmaceutical medication in the treatment of rheumatoid arthritis and soft-tissue rheumatism in the 1950s.[2] It is no longer used today.[4]
Available forms
Pregnenolone acetate was available as Enelone in the form of 100 mg oral tablets and as a 100 mg/mL crystalline aqueous suspension in 10 mL vials.[2]
Pharmacology
Pregnenolone is a neurosteroid.[5][6] It is a negative allosteric modulator of the CB1 receptor,[5][8] a ligand of the microtubule-associated protein 2 (MAP2),[9][10] and an agonist of the pregnane X receptor.[11] Pregnenolone has no progestogenic, corticosteroid, estrogenic, androgenic, or antiandrogenic activity.[3] In addition to its own activities, pregnenolone is a precursor for other neurosteroids such as pregnenolone sulfate, allopregnanolone, and pregnanolone and for steroid hormones.[12][13][14][15]
Pregnenolone has low bioavailability and is subject to high metabolism.[5] Oral administration of 50 or 100 mg pregnenolone has been found to have minimal or negligible effect on urinary levels of testosterone and testosterone metabolites, including of androsterone, etiocholanolone, 5β-androstanediol, androstadienol, and androstenol (and/or their conjugates), and this suggests that only a small amount of pregnenolone is converted into testosterone.[13][14] This is in accordance with findings on the conversion of DHEA into testosterone, in which only 1.5% of an oral dose of DHEA was found to be converted into testosterone.[13] In contrast to the androstanes, 50 or 100 mg oral pregnenolone has been found to significantly and in fact "strongly" increase urinary levels of the progesterone metabolites pregnanediol and pregnanolone (and/or their conjugates), whereas pregnanetriol was unaffected.[13][14] Unlike the case of oral administration, transdermal administration of 30 mg/day pregnenolone cream has not been found to affect urinary levels of metabolites of any other steroids, including of progesterone.[14] Intranasal administration of pregnenolone was found to have low bioavailability of around 23%.[5]
Sripada et al. reported that oral pregnenolone is preferentially metabolized into the neurosteroid allopregnanolone rather than into other steroids such as DHEA or cortisol.[15] In further research by their group, a single 400 mg dose of oral pregnenolone at 3 hours post-administration was found to result in a 3-fold elevation in serum levels of pregnenolone and a 7-fold increase in allopregnanolone levels.[15] Pregnanolone levels increased by approximately 60% while DHEA levels decreased non-significantly by approximately 5% and cortisol levels were not affected.[15] Another study found that allopregnanolone levels were increased by 3-fold at 2 hours post-administration following a single 400 mg oral dose of pregnenolone.[15]
In addition to allopregnanolone, pregnenolone acts as a prodrug of pregnenolone sulfate.[12] However, pregnenolone sulfate does not cross the blood–brain barrier.[16][17]
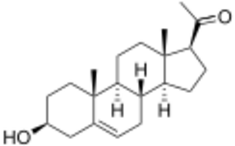
Chemistry
Pregnenolone, also known as 5-pregnenolone or as pregn-5-en-3β-ol-20-one, is a naturally occurring pregnane steroid and a derivative of cholesterol.[3][1][4] Related steroids include pregnenolone sulfate, 3β-dihydroprogesterone (4-pregnenolone), progesterone, allopregnanolone, and pregnanolone.[3][1][4]
Derivatives
A few synthetic ester derivatives of pregnenolone exist.[1] These include pregnenolone acetate (Antofin, Previsone, Pregno-Pan) and pregnenolone succinate (Panzalone, Formula 405).[1] Prebediolone acetate (Acetoxanon, Acetoxy-Prenolon, Artisone, Artivis, Pregnartrone, Sterosone), the 21-acetate ester of 21-hydroxypregnenolone, also exists.[1] These esters are all described as glucocorticoids similarly to pregnenolone.[1]
The 3β-methyl ether of pregnenolone, 3β-methoxypregnenolone (MAP-4343), retains similar activity to pregnenolone in regard to interaction with MAP2,[9][10] and is under development for potential clinical use for indications such as the treatment of brain and spinal cord injury and depressive disorders.[18][19][20][21]
History
Pregnenolone was first synthesized by Adolf Butenandt and colleagues in 1934.[3] It was first used in medicine, as an anti-inflammatory medication, in the 1940s.[5]
Society and culture
Generic names
Pregnenolone is the generic name of the drug and its INN, BAN, DCF, and JAN.[1][4][22]
Brand names
Pregnenolone has been marketed in the past under a variety of brand names including Arthenolone, Bina-Skin, Enelone, Natolone, Pregnetan, Pregneton, Pregnolon, Prenolon, Prenolone, Regnosone, Sharmone, and Skinostelon.[1][4]
Availability
Pregnenolone is no longer marketed as a medication, but remains available as a supplement.[4][23]
Alternative medicine
Pregnenolone has been promoted online with claims it can treat a variety of diseases including multiple sclerosis, arthritis, and cancer, but such claims are not backed by evidence.[7]
Research
(As of 2016) pregnenolone is being researched for possible therapeutic applications, but its poor bioavailability makes its prospects for usefulness low.[5] Pregnenolone is available as an over-the-counter supplement, for instance in the United States .[23]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 665–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA665.
- ↑ 2.0 2.1 2.2 2.3 2.4 Modern Drug Encyclopedia and Therapeutic Index. Yorke Medical Group. 1955. pp. 382–. https://books.google.com/books?id=IrXGAAAAIAAJ&pg=PA382.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Pregnenolone". The Journal of Clinical Endocrinology and Metabolism 10 (4): 455–474. April 1950. doi:10.1210/jcem-10-4-455. PMID 15415436.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 872–873. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA872.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Neurosteroids and potential therapeutics: Focus on pregnenolone". The Journal of Steroid Biochemistry and Molecular Biology 160: 78–87. June 2016. doi:10.1016/j.jsbmb.2015.09.030. PMID 26433186.
- ↑ 6.0 6.1 "Nongenomic actions of neurosteroid pregnenolone and its metabolites". Steroids 111: 54–59. July 2016. doi:10.1016/j.steroids.2016.01.017. PMID 26844377.
- ↑ 7.0 7.1 "Pregnenolone". American Cancer Society Complete Guide to Complementary and Alternative Cancer Therapies (2nd ed.). American Cancer Society. 2009. pp. 807-810. ISBN 9780944235713. https://archive.org/details/americancancerso0000unse/page/807.
- ↑ "Endocannabinoids and Their Pharmacological Actions" (in en). Endocannabinoids. Handbook of Experimental Pharmacology. 231. Springer International Publishing. 2015. pp. 1–37. doi:10.1007/978-3-319-20825-1_1. ISBN 9783319208244.
- ↑ 9.0 9.1 "Neurosteroid regulation of central nervous system development". Pharmacology & Therapeutics 116 (1): 107–124. October 2007. doi:10.1016/j.pharmthera.2007.04.011. PMID 17651807.
- ↑ 10.0 10.1 "Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor". Proceedings of the National Academy of Sciences of the United States of America 103 (12): 4711–4716. March 2006. doi:10.1073/pnas.0600113103. PMID 16537405. Bibcode: 2006PNAS..103.4711F.
- ↑ "The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways". Recent Progress in Hormone Research 54: 345–67; discussion 367–8. 1999. PMID 10548883.
- ↑ 12.0 12.1 "Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration". European Journal of Pharmacology 641 (2-3): 128–134. September 2010. doi:10.1016/j.ejphar.2010.05.033. PMID 20570588.
- ↑ 13.0 13.1 13.2 13.3 "Urinary marker of oral pregnenolone administration". Steroids 70 (3): 179–183. March 2005. doi:10.1016/j.steroids.2004.12.007. PMID 15763596.
- ↑ 14.0 14.1 14.2 14.3 "Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration". Drug Testing and Analysis 3 (5): 283–290. May 2011. doi:10.1002/dta.281. PMID 21538944.
- ↑ 15.0 15.1 15.2 15.3 15.4 "Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits". Biological Psychiatry 73 (11): 1045–1053. June 2013. doi:10.1016/j.biopsych.2012.12.008. PMID 23348009.
- ↑ "Impact of Neuroendocrine Factors on Seizure Genesis and Treatment". Epilepsy: Mechanisms, Models, and Translational Perspectives. CRC Press. 18 June 2010. pp. 479–. ISBN 978-1-4200-8560-0. https://books.google.com/books?id=8prMBQAAQBAJ&pg=PA479.
- ↑ CIBA Foundation Symposium (30 April 2008). Steroids and Neuronal Activity. John Wiley & Sons. pp. 101–. ISBN 978-0-470-51399-6. https://books.google.com/books?id=KeZlL3XbmQsC&pg=PA101.
- ↑ "Pregnenolone methyl ether". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800034216.
- ↑ "Treatment of experimental spinal cord injury with 3β-methoxy-pregnenolone". Brain Research 1403: 57–66. July 2011. doi:10.1016/j.brainres.2011.05.065. PMID 21704982.
- ↑ "3β-Methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders". Proceedings of the National Academy of Sciences of the United States of America 109 (5): 1713–1718. January 2012. doi:10.1073/pnas.1121485109. PMID 22307636. Bibcode: 2012PNAS..109.1713B.
- ↑ "From steroid hormones to depressive states and senile dementias: New mechanistic, therapeutical and predictive approaches". Comptes Rendus Biologies 338 (8-9): 613–616. 2015. doi:10.1016/j.crvi.2015.06.003. PMID 26251072.
- ↑ "145-13-1 - ORNBQBCIOKFOEO-QGVNFLHTSA-N - Pregnenolone [INN:BAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". ChemIDplus. U.S. National Library of Medicine. https://chem.nlm.nih.gov/chemidplus/rn/145-13-1.
- ↑ 23.0 23.1 "Chronic pregnenolone effects in normal humans: attenuation of benzodiazepine-induced sedation". Psychoneuroendocrinology 29 (4): 486–500. May 2004. doi:10.1016/S0306-4530(03)00056-8. PMID 14749094.
 |

