Chemistry:Remifentanilic acid
From HandWiki
Short description: Inactive metabolite of remifentanil
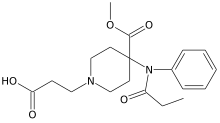 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H26N2O5 |
| Molar mass | 362.426 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Remifentanilic acid is a metabolite of the potent short-acting synthetic opioid analgesic drug remifentanil. It is an analog of fentanyl and remifentanil, but is not active as an opioid in its own right.[1][2][3][4][5][6][7][8]
See also
- 3-Methylbutyrfentanyl
- 4-Fluorobutyrfentanyl
- 4-Fluorofentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Benzylfentanyl
- Furanylfentanyl
- Homofentanyl
- List of fentanyl analogues
References
- ↑ Pitsiu, M. (2004). "Pharmacokinetics of remifentanil and its major metabolite, remifentanil acid, in ICU patients with renal impairment". British Journal of Anaesthesia 92 (4): 493–503. doi:10.1093/bja/aeh086. PMID 14766712.
- ↑ Battershill, Anna J. (2006). "Remifentanil". Drugs 66 (3): 365–385. doi:10.2165/00003495-200666030-00013. PMID 16526829.
- ↑ Kan, Randall E. (1998). "Intravenous remifentanil: placental transfer, maternal and neonatal effects". The Journal of the American Society of Anesthesiologists 88 (6): 1467–1474. doi:10.1097/00000542-199806000-00008. PMID 9637638.
- ↑ Glass, Peter SA (1999). "A review of the pharmacokinetics and pharmacodynamics of remifentanil". Anesthesia & Analgesia 89 (4S): 7. doi:10.1097/00000539-199910001-00003. PMID 10511072.
- ↑ Nan, Zhang (2020). "Spectral Analysis and Structural Identification of Remifentanil Acid". Guangpuxue Yu Guangpu Fenxi/Spectroscopy and Spectral Analysis: 2059-2065.
- ↑ Kuhlen, Ralf (2003). "Remifentanil for analgesia-based sedation in the intensive care unit". Critical Care 8 (1): 1-3.
- ↑ Owen, Medge D. (2002). "Prolonged intravenous remifentanil infusion for labor analgesia". Anesthesia & Analgesia 94 (4): 918-9, table of contents. doi:10.1097/00000539-200204000-00027. PMID 11916797.
- ↑ Riches, James R. (2012). "Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use". Journal of Analytical Toxicology 36 (9): 647–656. doi:10.1093/jat/bks078. PMID 23002178.
Further reading
- "Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues". Forensic Toxicology 26 (1): 1–5. June 2008. doi:10.1007/s11419-007-0039-1.
- "Fentanyl receptor assay. II. Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine". J Anal Toxicol 16 (1): 36–41. 1992. doi:10.1093/jat/16.1.36. PMID 1322477.
- "Evaluation of new compounds for opioid activity: 1987 annual report". NIDA Res. Monogr. 81: 543–90. 1988. PMID 3136388.
- "Dependence studies of new compounds in the rhesus monkey, rat, and mouse, 1987". NIDA Res. Monogr. 81: 485–542. 1988. PMID 3136386.
- "Carbon-13 nuclear magnetic resonance spectra of fentanyl analogs". Journal of Heterocyclic Chemistry 26 (3): 677–686. 2009. doi:10.1002/jhet.5570260329.
 |

