Chemistry:Norfentanyl
From HandWiki
Short description: Synthetic opioid analgesic metabolite and precursor
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
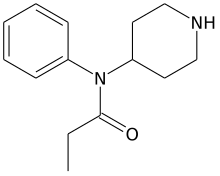
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Norfentanyl is an inactive synthetic opioid analgesic drug precursor.[1] It is an analog and metabolite of fentanyl with the removal of the phenethyl moiety (or functional group) from fentanyl chemical structure.[2][3][4][5][6][7][8][9]
Occurrence and Applications
Norfentanyl occurs primarily as a metabolite of its parent drug, fentanyl. However, it can also be used to synthesize fentanyl itself.
See also
- 3-Methylbutyrfentanyl
- 4-Fluorobutyrfentanyl
- 4-Fluorofentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Benzylfentanyl
- Furanylfentanyl
- Homofentanyl
- List of fentanyl analogues
References
- ↑ "Fentanyl". Elsevier. https://www.sciencedirect.com/topics/neuroscience/fentanyl.
- ↑ Sofalvi, Szabolcs (2017). "An LC–MS-MS method for the analysis of carfentanil, 3-methylfentanyl, 2-furanyl fentanyl, acetyl fentanyl, fentanyl and norfentanyl in postmortem and impaired-driving cases". Journal of Analytical Toxicology 41 (6): 473–483. doi:10.1093/jat/bkx052. PMID 28830122.
- ↑ Bista, Sudeep R. (2014). "Development, validation and application of an HPLC–MS/MS method for the determination of fentanyl and nor-fentanyl in human plasma and saliva.". Journal of Chromatography B (960): 27-33.
- ↑ Huynh, N-H. (2005). "Determination of fentanyl in human plasma and fentanyl and norfentanyl in human urine using LC–MS/MS". Journal of Pharmaceutical and Biomedical Analysis 37 (5): 1095–1100. doi:10.1016/j.jpba.2004.09.024. PMID 15862690.
- ↑ Poklis, Alphonse (2004). "Urine concentrations of fentanyl and norfentanyl during application of Duragesic® transdermal patches". Journal of Analytical Toxicology 28 (6): 422–425. doi:10.1093/jat/28.6.422. PMID 15516290.
- ↑ Coopman, Vera (2007). "LC–MS/MS analysis of fentanyl and norfentanyl in a fatality due to application of multiple Durogesic® transdermal therapeutic systems". Forensic Science International 169 (2–3): 223–227. doi:10.1016/j.forsciint.2006.03.018. PMID 16650707.
- ↑ Peer, Cody J. (2007). "Direct-injection mass spectrometric method for the rapid identification of fentanyl and norfentanyl in postmortem urine of six drug-overdose cases". Journal of Analytical Toxicology 31 (8): 515–521. doi:10.1093/jat/31.8.515. PMID 17988466.
- ↑ Vandergrift, Gregory W. (2018). "Paper spray mass spectrometry for the direct, semi-quantitative measurement of fentanyl and norfentanyl in complex matrices". Clinical Biochemistry 54: 106–111. doi:10.1016/j.clinbiochem.2018.02.005. PMID 29432758.
- ↑ Patton, Amy L. (2014). "Quantitative measurement of acetyl fentanyl and acetyl norfentanyl in human urine by LC-MS/MS". Analytical Chemistry 86 (3): 1760–1766. doi:10.1021/ac4036197. PMID 24354295.
Further reading
- "Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues". Forensic Toxicology 26 (1): 1–5. June 2008. doi:10.1007/s11419-007-0039-1.
- "Fentanyl receptor assay. II. Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine". J Anal Toxicol 16 (1): 36–41. 1992. doi:10.1093/jat/16.1.36. PMID 1322477.
- "Evaluation of new compounds for opioid activity: 1987 annual report". NIDA Res. Monogr. 81: 543–90. 1988. PMID 3136388.
- "Dependence studies of new compounds in the rhesus monkey, rat, and mouse, 1987". NIDA Res. Monogr. 81: 485–542. 1988. PMID 3136386.
- "Carbon-13 nuclear magnetic resonance spectra of fentanyl analogs". Journal of Heterocyclic Chemistry 26 (3): 677–686. 2009. doi:10.1002/jhet.5570260329.
 |

