Chemistry:Apimostinel
 | |
| Clinical data | |
|---|---|
| Other names | NRX-1074; AGN-241660; Threonyl-prolyl-2R-(2-benzyl)-prolyl-threonine amide |
| Routes of administration | By mouth |
| Drug class | NMDA receptor modulator |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
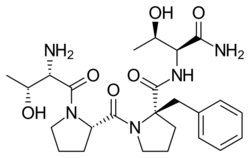
| Formula | C25H37N5O6 |
| Molar mass | 503.600 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Apimostinel (GATE-202, formerly NRX-1074) is an investigational antidepressant, acting as a novel and selective modulator of the NMDA receptor.[1][2][3][4] It is currently under development for the acute treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Naurex and Allergan.[5][6][7] As of February 2015, an intravenous formulation of apimostinel has completed a phase IIa clinical trial for MDD.[5][8]
Similar to rapastinel (GLYX-13), its mechanism of action acts through a unique binding site on the NMDA receptor, independent of the glycine site, to modulate receptor activity and enhance NMDAR-mediated synaptic plasticity.[9] However, apimostinel is 1000-fold more potent in vitro and is intended as an improved, follow-up drug to rapastinel.[2][5] Similar to rapastinel, apimostinel is an amidated tetrapeptide, but has been structurally modified, via the addition of a benzyl group, to enhance its metabolic stability and pharmacokinetic profile. The drug has shown rapid and potent antidepressant effects in pre-clinical models of depression.[5] In addition, similarly to rapastinel, it is well tolerated and lacks the schizophrenia-like psychotomimetic effects of NMDA receptor antagonists such as ketamine.[5]
See also
References
- ↑ "Naurex's Novel Antidepressant GLYX-13 Recognized as One of Windhover's Top 10 Neuroscience Projects to Watch". PR Newswire. 31 August 2010. http://www.prnewswire.com/news-releases/naurexs-novel-antidepressant-glyx-13-recognized-as-one-of-windhovers-top-10-neuroscience-projects-to-watch-101866728.html.
- ↑ 2.0 2.1 "Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status". CNS Drugs 35 (5): 527–543. May 2021. doi:10.1007/s40263-021-00816-x. PMID 33904154.
- ↑ "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology 22 (3): 247–259. March 2019. doi:10.1093/ijnp/pyy101. PMID 30544218.
- ↑ "Neuroplasticity and the next wave of antidepressant strategies". Frontiers in Cellular Neuroscience 7: 218. November 2013. doi:10.3389/fncel.2013.00218. PMID 24312008.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Naurex Reports Positive Top-Line Phase 2b Results for Novel Antidepressant GLYX-13 and Advances NRX-1074 into Phase 2 Depression Study". PR Newswire. 6 May 2014. http://www.prnewswire.com/news-releases/naurex-reports-positive-top-line-phase-2b-results-for-novel-antidepressant-glyx-13-and-advances-nrx-1074-into-phase-2-depression-study-258089291.html.
- ↑ "Allergan Successfully Completes Naurex Acquisition". Allergan plc (Press release). PR Newswire. Retrieved 2016-11-20.
- ↑ "Home - Gate Neurosciences" (in en-US). https://www.gateneuro.com/.
- ↑ Clinical trial number NCT02067793 for "Study of Intravenous NRX-1074 in Patients With Major Depressive Disorder" at ClinicalTrials.gov
- ↑ "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology 22 (3): 247–259. March 2019. doi:10.1093/ijnp/pyy101. PMID 30544218.
External links
 |

