Chemistry:Estrone sulfate (medication)
 | |
 | |
| Clinical data | |
|---|---|
| Other names | E1S; Oestrone sulfate; Estrone 3-sulfate; Estra-1,3,5(10)-trien-17-one 3-sulfate |
| Routes of administration | By mouth, others[1][2][3] |
| Drug class | Estrogen; Estrogen ester |
| Pharmacokinetic data | |
| Metabolism | Desulfation (via STS)[5] |
| Metabolites | • Estrone[1] • Estradiol[1] |
| Elimination half-life | 12 hours[4] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H22O5S |
| Molar mass | 350.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone.[1] It is used in menopausal hormone therapy among other indications.[1][2] As the sodium salt (sodium estrone sulfate), it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens (Estratab, Menest).[1][3] In addition, E1S is used on its own as the piperazine salt estropipate (piperazine estrone sulfate; Ogen).[1][3] The compound also occurs as a major and important metabolite of estradiol and estrone.[1] E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.[1][2]
Medical uses
E1S is used in menopausal hormone therapy among other indications.[1][2]
Pharmacology
Pharmacodynamics
E1S itself is essentially biologically inactive, with less than 1% of the relative binding affinity of estradiol for the estrogen receptors (ERs), ERα and ERβ.[6] The compound acts as a prodrug of estrone and more importantly of estradiol, the latter of which is a potent agonist of the ERs.[1] Hence, E1S is an estrogen.[1]
Pharmacokinetics
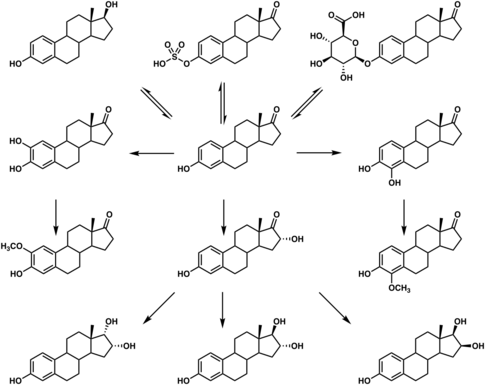
E1S is cleaved by steroid sulfatase (also called estrogen sulfatase) into estrone.[5] Simultaneously, estrogen sulfotransferases transform estrone back into E1S, which results in an equilibrium between the two steroids in various tissues.[5] E1S is thought to serve both as a rapidly-acting prodrug of estradiol and also as a long-lasting reservoir of estradiol in the body, which serves to greatly extend the duration of estradiol when used as a medication.[1][7][8]
When estradiol is administered orally, it is subject to extensive first-pass metabolism (95%) in the intestines and liver.[9][10] A single administered dose of estradiol is absorbed 15% as estrone, 25% as E1S, 25% as estradiol glucuronide, and 25% as estrone glucuronide.[9] Formation of estrogen glucuronide conjugates is particularly important with oral estradiol as the percentage of estrogen glucuronide conjugates in circulation is much higher with oral ingestion than with parenteral estradiol.[9] Estrone glucuronide can be reconverted back into estradiol, and a large circulating pool of estrogen glucuronide and sulfate conjugates serves as a long-lasting reservoir of estradiol that effectively extends its terminal half-life of oral estradiol.[9][10] To demonstrate the importance of first-pass metabolism and the estrogen conjugate reservoir in the pharmacokinetics of estradiol,[9] the terminal half-life of oral estradiol is 13 to 20 hours[11] whereas with intravenous injection its terminal half-life is only about 1 to 2 hours.[12]
Estrogen sulfates like estrone sulfate are about twice as potent as the corresponding free estrogens in terms of estrogenic effect when given orally to rodents.[13] This in part led to the introduction of conjugated estrogens (Premarin), which are primarily estrone sulfate, in 1941.[13]
Chemistry
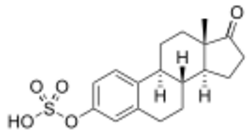
E1S, also known as estrone 3-sulfate or as estra-1,3,5(10)-trien-17-one 3-sulfate, is a naturally occurring estrane steroid and a derivative of estrone.[17] It is an estrogen conjugate or ester, and is specifically the C3 sulfate ester of estrone.[17] Salts of E1S include sodium estrone sulfate and estropipate (piperazine estrone sulfate).[17][1][3]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cder/daf/.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Pharmacology for Women's Health. Jones & Bartlett Publishers. 8 September 2015. pp. 361–. ISBN 978-1-284-05748-5. https://books.google.com/books?id=AniUCgAAQBAJ&pg=PA361.
- ↑ Brody's Human Pharmacology. Elsevier Health Sciences. 1 April 2009. pp. 456–. ISBN 978-0-323-07575-6. https://books.google.com/books?id=kfsrz_-OrMQC&pg=PA456.
- ↑ Jump up to: 5.0 5.1 5.2 Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. 22 May 2013. pp. 5–6. ISBN 978-1-4614-6837-0. https://books.google.com/books?id=TAYnR1b8jRkC&pg=PA5.
- ↑ "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology 138 (3): 863–870. March 1997. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ↑ Williams Textbook of Endocrinology (13th ed.). Elsevier Health Sciences. 11 November 2015. pp. 607–. ISBN 978-0-323-34157-8. https://books.google.com/books?id=iPIACwAAQBAJ&pg=PA607.
- ↑ Integrative Therapies for Depression: Redefining Models for Assessment, Treatment and Prevention. CRC Press. 27 April 2016. pp. 198–. ISBN 978-1-4987-0230-0. https://books.google.com/books?id=GpHwCgAAQBAJ&pg=PA198.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. 6 December 2012. pp. 268–. ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA268.
- ↑ Jump up to: 10.0 10.1 Current Management of the Menopause. CRC Press. 22 June 2005. pp. 364–. ISBN 978-0-203-48612-2. https://books.google.com/books?id=WD7S7677xUUC&pg=PA364.
- ↑ "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception 87 (6): 706–727. June 2013. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ↑ "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas 4 (4): 315–324. December 1982. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ↑ Jump up to: 13.0 13.1 "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. Springer. 1970. pp. 368–408. doi:10.1007/978-3-642-95177-0_8. ISBN 978-3-642-95179-4.
- ↑ The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. 2012. p. 64. ISBN 9781461255253. https://books.google.com/books?id=z0LuBwAAQBAJ&pg=PA64%7Cdate=6#v=onepage&q&f=false.
- ↑ "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society 8 Suppl 1: 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens. Technische Universität Braunschweig. January 2018. https://www.brenda-enzymes.org/enzyme.php?ecno=2.4.1.17&Suchword=estrone&reference=&UniProtAcc=&organism%5B%5D=Homo+sapiens. Retrieved 10 August 2018.
- ↑ Jump up to: 17.0 17.1 17.2 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 900–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA900.
- ↑ "Estrone-3-sulphate, a potential novel ligand for targeting breast cancers". PLOS ONE 8 (5): e64069. 2013. doi:10.1371/journal.pone.0064069. PMID 23717534. Bibcode: 2013PLoSO...864069B.
Further reading
- "Clinical implications of estrone sulfate measurement in laboratory medicine". Critical Reviews in Clinical Laboratory Sciences 54 (2): 73–86. March 2017. doi:10.1080/10408363.2016.1252310. PMID 27960570.
 |