Chemistry:Doisynoestrol
From HandWiki
Short description: Chemical compound
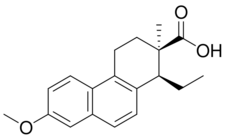 | |
| Clinical data | |
|---|---|
| Trade names | Fenocyclin, Surestrine, Surestryl |
| Other names | Diosynestrol; Fenocycline; Fenocyclin; Phenocyclin; RS-2874; Dehydrofolliculinic acid; cis-Bisdehydrodoisynolic acid 7-methyl ether; BDDA ME; NSC-56846; NSC-122041 |
| Routes of administration | By mouth |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H22O3 |
| Molar mass | 298.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Doisynoestrol (brand names Fenocyclin, Surestrine, Surestryl; former developmental code name RS-2874), also known as fenocycline, as well as cis-bisdehydrodoisynolic acid 7-methyl ether (BDDA ME), is a synthetic nonsteroidal estrogen of the doisynolic acid group that is no longer marketed.[1][2] It is a methyl ether of bisdehydrodoisynolic acid.[1] Doisynoestrol was described in the literature in 1945.[1] It has about 0.02% of the relative binding affinity of estradiol for the estrogen receptor.[3]
See also
References
- ↑ 1.0 1.1 1.2 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 465–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA465.
- ↑ "Separation of growth inhibiting potency from oestrogenicity in different weak oestrogenic drugs of various chemical structures". Acta Endocrinologica 68 (2): 249–63. October 1971. doi:10.1530/acta.0.0680249. PMID 5171465.
- ↑ "The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands". Toxicol Sci 54 (1): 138–53. March 2000. doi:10.1093/toxsci/54.1.138. PMID 10746941.
 |

