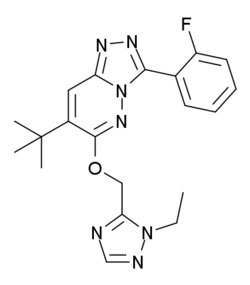
Chemistry:TPA-023
 | |
 | |
| Clinical data | |
|---|---|
| Other names | MK-0777 |
| Routes of administration | By mouth |
| Pharmacokinetic data | |
| Metabolism | liver |
| Elimination half-life | 6.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H22FN7O |
| Molar mass | 395.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
TPA-023 (MK-0777) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. It is a mixed, subtype-selective ligand of the benzodiazepine site of α1, α2, α3, and α5-containing GABAA receptors, where it acts as a partial agonist at benzodiazepine sites of the α2 and α3-containing subtypes, but as a silent antagonist at α1 and α5-containing subtypes.[1] It has primarily anxiolytic and anticonvulsant effects in animal tests, but with no sedative effects even at 50 times the effective anxiolytic dose.[2][3]
In human trials on healthy volunteers, TPA-023 was comparable to lorazepam, but had much less side effects on cognition, memory, alertness or coordination.[4] In Phase II trials, the compound was significantly superior to placebo without inducing sedation. The clinical development was halted due to preclinical toxicity (cataract) in long term dosing studies.[5][6] TPA-023 is well absorbed following oral administration and extensively metabolised by the liver, with a half-life of 6.7 hours.[7] The main enzyme involved in its metabolism is CYP3A4, with some contribution by CYP3A5.[8]
References
- ↑ "Novel discriminative stimulus effects of TPA023B, subtype-selective gamma-aminobutyric-acid(A)/benzodiazepine modulator: comparisons with zolpidem, lorazepam, and TPA023". Pharmacology, Biochemistry, and Behavior 90 (1): 65–73. July 2008. doi:10.1016/j.pbb.2008.02.019. PMID 18395780.
- ↑ "7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine: a functionally selective gamma-aminobutyric acid(A) (GABA(A)) alpha2/alpha3-subtype selective agonist that exhibits potent anxiolytic activity but is not sedating in animal models". Journal of Medicinal Chemistry 48 (23): 7089–92. November 2005. doi:10.1021/jm058034a. PMID 16279764.
- ↑ "TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates". The Journal of Pharmacology and Experimental Therapeutics 316 (1): 410–22. January 2006. doi:10.1124/jpet.105.089920. PMID 16183706.
- ↑ "Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers". Journal of Psychopharmacology 21 (4): 374–83. June 2007. doi:10.1177/0269881106072343. PMID 17092968.
- ↑ "The rise of a new GABA pharmacology". Neuropharmacology 60 (7–8): 1042–9. June 2011. doi:10.1016/j.neuropharm.2010.10.020. PMID 21035473.
- ↑ "GABA(A) receptor subtype-selective efficacy: TPA023, an alpha2/alpha3 selective non-sedating anxiolytic and alpha5IA, an alpha5 selective cognition enhancer". CNS Neuroscience & Therapeutics 14 (1): 25–35. 2008. doi:10.1111/j.1527-3458.2007.00034.x. PMID 18482097.
- ↑ "Metabolism and disposition of a potent and selective GABA-Aalpha2/3 receptor agonist in healthy male volunteers". Drug Metabolism and Disposition 34 (6): 1004–11. June 2006. doi:10.1124/dmd.105.008193. PMID 16510541.
- ↑ "Cytochrome P450 3A-dependent metabolism of a potent and selective gamma-aminobutyric acid Aalpha2/3 receptor agonist in vitro: involvement of cytochrome P450 3A5 displaying biphasic kinetics". Drug Metabolism and Disposition 35 (8): 1301–7. August 2007. doi:10.1124/dmd.107.014753. PMID 17460031.
 |

