Chemistry:6α-Methylprogesterone
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | 6α-Methylpregn-4-ene-3,20-dione; 6α-MP; 6-MP; 6MP; NSC-75530 |
| Drug class | Progestogen; Progestin |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
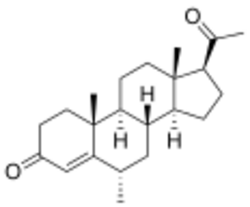
| Formula | C22H32O2 |
| Molar mass | 328.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
6α-Methylprogesterone (6α-MP) is a progestin which was never marketed.[1][2][3][4][5][6][7] It has 150% of the progestogenic potency of progesterone.[5] In addition, and in contrast to progesterone, 6α-MP has weak androgenic, antiandrogenic, and synandrogenic actions.[5] 6α-MP is structurally related to medroxyprogesterone acetate (MPA; 6α-methyl-17α-acetoxyprogesterone) and megestrol acetate (MGA; 6-dehydro-6-methyl-17α-acetoxyprogesterone), which possess androgenic and/or antiandrogenic activity to varying degrees similarly.[5] MPA is more androgenic than 6α-MP and MGA.[5]
References
- ↑ "Synthesis of 6-alpha-methylprogesterone from hyodesoxycholic acid". Scientia Sinica 14 (10): 1533–1535. October 1965. PMID 5881565.
- ↑ "In Vivo Metabolism and Binding of 6α-Methylprogesterone; A Progestin with Anti-Androgenic and Synandrogenic Activities". Steroid Hormone Receptor Systems. Advances in Experimental Medicine and Biology. 117. 1979. pp. 269–280. doi:10.1007/978-1-4757-6589-2_14. ISBN 978-1-4757-6591-5.
- ↑ "The nuclear uptake of 6 alpha-[3H]methylprogesterone and its 20 alpha-hydroxy metabolite: the requirement for multiple receptors". Endocrinology 109 (6): 1821–1829. December 1981. doi:10.1210/endo-109-6-1821. PMID 7308133.
- ↑ "The biological actions and metabolism of 6 alpha-methylprogesterone: a progestin that mimics and modifies the effects of testosterone". Endocrinology 109 (6): 1814–1820. December 1981. doi:10.1210/endo-109-6-1814. PMID 7308132.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Progestins can mimic, inhibit and potentiate the actions of androgens". Pharmacology & Therapeutics 23 (3): 443–459. 1983. doi:10.1016/0163-7258(83)90023-2. PMID 6371845.
- ↑ "Receptor binding of 6 alpha-methylprogesterone in mouse kidney". Hormone Research 21 (4): 261–269. 1985. doi:10.1159/000180059. PMID 4007785.
- ↑ "Steroids of One Class Can Mimic, Inhibit and Potentiate the Biological Effects of Other Steroid Classes when Administered at High Doses". Contraceptive Steroids. 1986. pp. 123–143. doi:10.1007/978-1-4613-2241-2_6. ISBN 978-1-4612-9313-2.
 |

