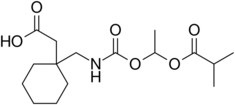
Chemistry:Gabapentin enacarbil
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Horizant, Regnite |
| Other names | XP-13512 |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Gabapentinoid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C16H27NO6 |
| Molar mass | 329.393 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gabapentin enacarbil (Horizant (ER) (U.S.), Regnite (in Japan )) is an anticonvulsant and analgesic drug of the gabapentinoid class, and a prodrug to gabapentin.[1] It was designed for increased oral bioavailability over gabapentin,[2][3] and human trials showed it to produce extended release of gabapentin with almost twice the overall bioavailability,[4] especially when taken with a fatty meal.[5] Gabapentin enacarbil has passed human clinical trials for the treatment of restless legs syndrome, and initial results have shown it to be well tolerated and reasonably effective.[6][7][8]
Gabapentin enacarbil was denied approval by the U.S. Food and Drug Administration (FDA) in February 2010, citing concerns about possible increased cancer risk shown by some animal studies. Similar concerns had been raised about gabapentin itself in the past, but were felt to be outweighed by its clinical utility as an anticonvulsant, whereas the treatment of restless legs syndrome was not seen to justify the same kind of risk.[9] On April 6, 2011, Xenoport received FDA approval for Horizant (gabapentin enacarbil) for the treatment of moderate-to-severe restless legs syndrome.[10] On June 7, 2012, the FDA approved Horizant for the treatment of postherpetic neuralgia in adults.[11]
References
- ↑ "Modifications of antiepileptic drugs for improved tolerability and efficacy". Perspectives in Medicinal Chemistry 2: 21–39. 2008. doi:10.1177/1177391X0800200001. PMID 19787095.
- ↑ "XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters". The Journal of Pharmacology and Experimental Therapeutics 311 (1): 315–23. October 2004. doi:10.1124/jpet.104.067934. PMID 15146028.
- ↑ "XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys". The Journal of Pharmacology and Experimental Therapeutics 311 (1): 324–33. October 2004. doi:10.1124/jpet.104.067959. PMID 15146029.
- ↑ "Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin". Journal of Clinical Pharmacology 48 (12): 1378–88. December 2008. doi:10.1177/0091270008322909. PMID 18827074.
- ↑ "The effect of food with varying fat content on the clinical pharmacokinetics of gabapentin after oral administration of gabapentin enacarbil". International Journal of Clinical Pharmacology and Therapeutics 48 (2): 120–8. February 2010. doi:10.5414/cpp48120. PMID 20137764.
- ↑ "Gabapentin enacarbil in restless legs syndrome". Drugs of Today 46 (1): 3–11. January 2010. doi:10.1358/dot.2010.46.1.1424766. PMID 20200691.
- ↑ "Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study". Mayo Clinic Proceedings 85 (6): 512–21. June 2010. doi:10.4065/mcp.2009.0700. PMID 20511481.
- ↑ "Gabapentin enacarbil (XP13512/GSK1838262) as an alternative treatment to dopaminergic agents for restless legs syndrome". Expert Opinion on Pharmacotherapy 11 (11): 1925–32. August 2010. doi:10.1517/14656566.2010.494598. PMID 20629607.
- ↑ GlaxoSmithKline/XenoPort: FDA setback halts gabapentin reformulations[yes|permanent dead link|dead link}}]
- ↑ "XenoPort, Inc. > Investors > RSS Content". http://phx.corporate-ir.net/phoenix.zhtml?c=187883&p=RssLanding&cat=news&id=1547555.
- ↑ Jeffrey, Susan. "FDA Approves Gabapentin Enacarbil for Postherpetic Neuralgia". Medscape. http://www.medscape.com/viewarticle/765233.
External links
- "Gabapentin enacarbil". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/gabapentin%20enacarbil.
 |

