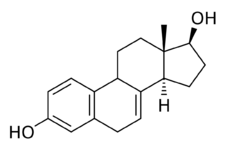
Chemistry:17β-Dihydroequilin
 | |
| Clinical data | |
|---|---|
| Other names | β-Dihydroequilin; Δ7-17β-Estradiol; 7-Dehydro-17β-estradiol; Estra-1,3,5(10),7-tetraen-3,17β-diol; NSC-12170 |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
17β-Dihydroequilin is a naturally occurring estrogen sex hormone found in horses as well as a medication.[1][2] As the C3 sulfate ester sodium salt, it is a minor constituent (1.7%) of conjugated estrogens (CEEs; brand name Premarin).[1] However, as equilin, with equilin sulfate being a major component of CEEs, is transformed into 17β-dihydroequilin in the body, analogously to the conversion of estrone into estradiol, 17β-dihydroequilin is, along with estradiol, the most important estrogen responsible for the effects of CEEs.[1]
Pharmacology
Pharmacodynamics
17β-Dihydroequilin is an estrogen, or an agonist of the estrogen receptors (ERs), the ERα and ERβ.[1] In terms of relative binding affinity for the ERs, 17β-dihydroequilin has about 113% and 108% of that of estradiol for the ERα and ERβ, respectively.[1] 17β-Dihydroequilin has about 83% of the relative potency of CEEs in the vagina and 200% of the relative potency of CEEs in the uterus.[1] Of the equine estrogens, it shows the highest estrogenic activity and greatest estrogenic potency.[1]
Like CEEs as a whole, 17β-dihydroequilin has disproportionate effects in certain tissues such as the liver and uterus.[1] Equilin, the second major component of conjugated estrogens after estrone, is reversibly transformed into 17β-dihydroequilin analogously to the transformation of estrone into estradiol.[1] However, whereas the balance of mutual interconversion of estrone and estradiol is largely shifted in the direction of estrone, it is nearly equal in the case of equilin and 17β-dihydroequilin.[1] As such, although 17β-dihydroequilin is only a minor constituent of CEEs, it is, along with estradiol, the most important estrogen relevant to the estrogenic activity of the medication.[1]
Pharmacokinetics
17β-Dihydroequilin has about 30% of the relative binding affinity of testosterone for sex hormone-binding globulin (SHBG), relative to 50% for estradiol.[1] The metabolic clearance rate of 17β-dihydroequilin is 1,250 L/day/m2, relative to 580 L/day/m2 for estradiol.[1]
Chemistry
17β-Dihydroequilin, or simply β-dihydroequilin, also known as δ7-17β-estradiol or as 7-dehydro-17β-estradiol, as well as estra-1,3,5(10),7-tetraen-3,17β-diol, is a naturally occurring estrane steroid and an analogue of estradiol.[1] In terms of chemical structure and pharmacology, equilin (δ7-estrone) is to 17β-dihydroequilin as estrone is to estradiol.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf.
- ↑ "Postmenopausal Hormone Therapy". Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. 28 March 2012. pp. 751–. ISBN 978-1-4511-4847-3. https://books.google.com/books?id=KZLubBxJEwEC&pg=PA751.
 |

