(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description: Chemical compound
CP-1414S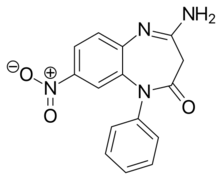 |
| Clinical data |
|---|
| ATC code | |
|---|
| Identifiers |
|---|
1-phenyl-4-amino-8-nitro-3H-1,5-benzodiazepin-2-one
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| Chemical and physical data |
|---|
| Formula | C15H12N4O3 |
|---|
| Molar mass | 296.286 g·mol−1 |
|---|
InChI=1S/C15H12N4O3/c16-14-9-15(20)18(10-4-2-1-3-5-10)13-8-11(19(21)22)6-7-12(13)17-14/h1-8H,9H2,(H2,16,17)  Y YKey:MBSVPZTUXNRICW-UHFFFAOYSA-N  Y Y
|
| (verify) |
CP-1414S is an experimental drug first made by a team in Germany.[1] It is a benzodiazepine derivative. CP-1414S is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring, and so is most closely related to other 1,5-benzodiazepines such as clobazam.
CP-1414S has primarily anxiolytic and anticonvulsant effects.[2] Its potency is roughly equal to that of clobazam, but with more pronounced sedation.[3]
See also
References
- ↑ US Patent 3766169 PROCESS FOR THE PREPARATION OF 3-AMINOMETHYLIDENE-1,5-BENZODIAZEPINE-2,4-(3H,5H)-DIONES
- ↑ "Studies on some pharmacological activities of 7-nitro-2-amino-5-phenyl-3H-1,5-benzodiazepine (CP 1414 S) in the rat. A comparison with diazepam". Arzneimittel-Forschung 31 (10): 1721–3. 1981. PMID 6119091.
- ↑ "Benzodiazepine receptors': correlation with pharmacological responses in living animals". Life Sciences 31 (19): 2025–35. November 1982. doi:10.1016/0024-3205(82)90094-7. PMID 6129557.
|
|---|
| Alcohols | |
|---|
| Barbiturates | |
|---|
| Benzodiazepines | |
|---|
| Carbamates | |
|---|
| Flavonoids | |
|---|
| Imidazoles | |
|---|
| Kava constituents | |
|---|
| Monoureides | |
|---|
| Neuroactive steroids | |
|---|
| Nonbenzodiazepines | |
|---|
| Phenols | |
|---|
| Piperidinediones | |
|---|
| Pyrazolopyridines | |
|---|
| Quinazolinones | |
|---|
| Volatiles/gases | |
|---|
| Others/unsorted |
- 3-Hydroxybutanal
- α-EMTBL
- AA-29504
- Avermectins (e.g., ivermectin)
- Bromide compounds (e.g., lithium bromide, potassium bromide, sodium bromide)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- DEABL
- Dihydroergolines (e.g., dihydroergocryptine, dihydroergosine, dihydroergotamine, ergoloid (dihydroergotoxine))
- DS2
- Efavirenz
- Etazepine
- Etifoxine
- Fenamates (e.g., flufenamic acid, mefenamic acid, niflumic acid, tolfenamic acid)
- Fluoxetine
- Flupirtine
- Hopantenic acid
- Lanthanum
- Lavender oil
- Lignans (e.g., 4-O-methylhonokiol, honokiol, magnolol, obovatol)
- Loreclezole
- Menthyl isovalerate (validolum)
- Monastrol
- Niacin
- Nicotinamide (niacinamide)
- Org 25,435
- Phenytoin
- Propanidid
- Retigabine (ezogabine)
- Safranal
- Seproxetine
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal), tetronal, trional)
- Terpenoids (e.g., borneol)
- Topiramate
- Valerian constituents (e.g., isovaleric acid, isovaleramide, valerenic acid, valerenol)
|
|---|
|
 | Original source: https://en.wikipedia.org/wiki/CP-1414S. Read more |



