Chemistry:Uldazepam
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
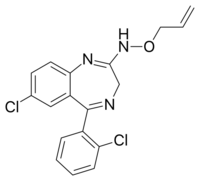
| Other names | 7-chloro-5-(2-chlorophenyl)-N-prop-2-enoxy-3H-1,4-benzodiazepin-2-amine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H15Cl2N3O |
| Molar mass | 360.24 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Uldazepam is a drug which is a benzodiazepine derivative.[1] It has sedative and anxiolytic effects similar to those of other benzodiazepines.[2][3]
Synthesis
Thio thionamide is even more prone to amidine formation than the lactam itself.
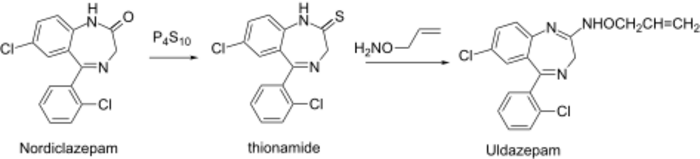
Uldazepam synthesis:[4] J. B. Hester, Jr., (1970); Chem. Abstr., 73: 99,001t (1970).
Reaction of thionamide (2) with O-allyl-hydroxylamine gave the oximino (3) uldazepam.
See also
References
- ↑ "Uldazepam U 31920". Psychotropics. http://www.psychotropics.dk/moleculeView/default.aspx?ID=1433&Catalogtype=A&ChapterID=1&Thissortorder=30.
- ↑ "[Mechanism of the polarographic reduction of the tranquilizer uldazepam]" (in de). Archiv der Pharmazie 321 (2): 69–72. February 1988. doi:10.1002/ardp.19883210205. PMID 3369929.
- ↑ "Clinical and computerized EEG effects of U-31,920, a new anxiolytic". Current Therapeutic Research, Clinical and Experimental 16 (6): 642–54. June 1974. PMID 4211146.
- ↑ Hester Jr., Jackson Boling, "3H-1,4-benzodiazepine und Verfahren zu deren Herstellung [3H-1,4-benzodiazepines and processes for their preparation]", DE patent 2005176, published 1970-09-10
 |

