Chemistry:Levacetylmethadol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | OrLAAM |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ~80% |
| Metabolism | CYP3A4 |
| Elimination half-life | 2.6 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
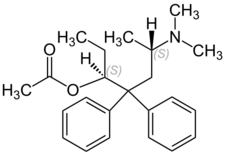
| Formula | C23H31NO2 |
| Molar mass | 353.498 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Levacetylmethadol (INN), levomethadyl acetate (USAN), OrLAAM (trade name) or levo-α-acetylmethadol (LAAM)[1][2] is a synthetic opioid similar in structure to methadone. It has a long duration of action due to its active metabolites.
Medical uses
LAAM is indicated as a second-line treatment for the treatment and management of opioid dependence if patients fail to respond to drugs like methadone or buprenorphine.
LAAM is used as an oral solution of LAAM hydrochloride at a concentration of 10 mg/mL in bottles of 120 and 500 mL under the brand name Orlaam. The first dose of LAAM for patients who have not started treatment with methadone is 20–40 mg. The first dose for patients who have been receiving methadone will be a little higher than the amount of methadone that was being taken every day, but not more than 120 mg. Afterwards, the dosage may be adjusted as needed. Unlike methadone, which requires daily administration, LAAM is administered two to three times a week.
Pharmacology
Pharmacodynamics
LAAM acts as a μ-opioid receptor agonist.
It also acts as a potent, noncompetitive α3β4 neuronal nicotinic acetylcholine receptor antagonist.[3]
Pharmacokinetics
LAAM undergoes extensive first-pass metabolism to the active demethylated metabolite nor-LAAM, which is further demethylated to a second active metabolite, dinor-LAAM. These metabolites are more potent than the parent drug.
Chemistry
LAAM, or levomethadyl acetate, is the levo isomer of acetylmethadol, or α-methadyl acetate. The dextro isomer, d-alphacetylmethadol (d-α-acetylmethadol), is more potent but shorter acting. The levo isomer is also less toxic with an -1">50 in mice of 110 mg/kg s.c. and 172.8 mg/kg orally as opposed to -1">50s of 61 mg/kg s.c. and 118.3 mg/kg orally for dl-α-methadyl acetate. It has a melting point of 215 °C and a molecular weight of 353.50. β-methadyl acetate also exists, however it is more toxic and less active than α-methadyl acetate and has no current medical use.
History
LAAM was approved in 1993 by the U.S. Food and Drug Administration for use in the treatment of opioid dependence. In 2001, LAAM was removed from the European market due to reports of life-threatening ventricular rhythm disorders.[4] In 2003, Roxane Laboratories, Inc. discontinued Orlaam in the US.[5]
Society and culture
Legal status
Before August 1993, LAAM was classified as a schedule I drug in the United States. LAAM is not approved for use in Australia and Canada . At present, it is a Schedule II Narcotic controlled substance in the United States with a DEA ACSCN of 9648 and a national aggregate annual manufacturing quota of 4 grammes as of 2013.
References
- ↑ Pholand A, "3-Acetoxy-4,4-diphenyl-6-methylaminoheptane", US patent 3021360, issued 12 February 1962, assigned to Eli Lilly and Company
- ↑ Clark RL, "Alpha-d1-4-acetoxy-1-methyl-3,3-diphenylhexylamine and salts", US patent 2565592, issued 28 August 1951, assigned to Merck & Company
- ↑ "Blockade of rat α3β4 nicotinic receptor function by methadone, its metabolites, and structural analogs". The Journal of Pharmacology and Experimental Therapeutics 299 (1): 366–71. October 2001. PMID 11561100. http://jpet.aspetjournals.org/content/299/1/366.short.
- ↑ "EMEA Public Statement on the Recommendation to Suspend the Marketing Authorisation for Orlaam (Levacetylmethadol) in the European Union". The European Agency for the Evaluation of Medicinal Products. 19 April 2001. http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2009/12/WC500018335.pdf.
- ↑ "Orlaam (levomethadyl acetate hydrochloride)". US FDA Safety Alerts. 20 August 2013. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm153332.htm.
Further reading
- "Dose-related efficacy of levomethadyl acetate for treatment of opioid dependence. A randomized clinical trial". JAMA 277 (24): 1945–51. June 1997. doi:10.1001/jama.1997.03540480045037. PMID 9200635.
- "Induction with levomethadyl acetate: safety and efficacy.". Archives of General Psychiatry 55 (8): 729–36. August 1998. doi:10.1001/archpsyc.55.8.729. PMID 9707384.
External links
- "LAAM Drug Information". Drugs.com. https://www.drugs.com/cons/levomethadyl.html.
- "Monograph for Orlaam". RxList. http://www.rxlist.com/cgi/generic2/levomethadyl_wcp.htm.
- "Levomethadyl Acetate". DrugBank. http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00745.txt.
 |

