(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description: Chemical compound
Lofendazam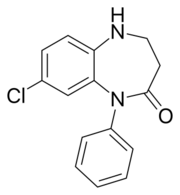 |
| Clinical data |
|---|
| ATC code | |
|---|
| Identifiers |
|---|
8-chloro-1-phenyl-1,3,4,5-tetrahydro- 2H-1,5-benzodiazepin- 2-one
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| Chemical and physical data |
|---|
| Formula | C15H13ClN2O |
|---|
| Molar mass | 272.73 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
Clc3ccc1c(N(C(=O)CCN1)c2ccccc2)c3
|
InChI=1S/C15H13ClN2O/c16-11-6-7-13-14(10-11)18(15(19)8-9-17-13)12-4-2-1-3-5-12/h1-7,10,17H,8-9H2  Y YKey:IUJQOUHDFKALCY-UHFFFAOYSA-N  Y Y
|
| (verify) |
Lofendazam[1] is an organic molecule which is a benzodiazepine derivative. Lofendazam is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring; therefore, lofendazam is most closely related to other 1,5-benzodiazepines such as clobazam.[2][3]
Lofendazam as a human pharmaceutical has sedative and anxiolytic effects similar to those produced by other benzodiazepine derivatives. It is an active metabolite of another benzodiazepine, arfendazam.[4]
See also
References
- ↑ DE patent 1929656
- ↑ "Syntheses and CD studies of optically active substituted 1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-ones.". Liebigs Annalen der Chemie 1995 (10): 1861–1869. 1995. doi:10.1002/jlac.1995199510261.
- ↑ "Nuclear magnetic resonance spectra of psychotherapeutic agents. V* - conformational analysis of 1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-ones.". Organic Magnetic Resonance 15 (4): 394–398. 1981. doi:10.1002/mrc.1270150414.
- ↑ The Pharmacology of Sleep. Springer. 11 December 1995. ISBN 978-3-540-58961-7.
|
|---|
| Alcohols | |
|---|
| Barbiturates | |
|---|
| Benzodiazepines | |
|---|
| Carbamates | |
|---|
| Flavonoids | |
|---|
| Imidazoles | |
|---|
| Kava constituents | |
|---|
| Monoureides | |
|---|
| Neuroactive steroids | |
|---|
| Nonbenzodiazepines | |
|---|
| Phenols | |
|---|
| Piperidinediones | |
|---|
| Pyrazolopyridines | |
|---|
| Quinazolinones | |
|---|
| Volatiles/gases | |
|---|
| Others/unsorted |
- 3-Hydroxybutanal
- α-EMTBL
- AA-29504
- Avermectins (e.g., ivermectin)
- Bromide compounds (e.g., lithium bromide, potassium bromide, sodium bromide)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- DEABL
- Dihydroergolines (e.g., dihydroergocryptine, dihydroergosine, dihydroergotamine, ergoloid (dihydroergotoxine))
- DS2
- Efavirenz
- Etazepine
- Etifoxine
- Fenamates (e.g., flufenamic acid, mefenamic acid, niflumic acid, tolfenamic acid)
- Fluoxetine
- Flupirtine
- Hopantenic acid
- Lanthanum
- Lavender oil
- Lignans (e.g., 4-O-methylhonokiol, honokiol, magnolol, obovatol)
- Loreclezole
- Menthyl isovalerate (validolum)
- Monastrol
- Niacin
- Nicotinamide (niacinamide)
- Org 25,435
- Phenytoin
- Propanidid
- Retigabine (ezogabine)
- Safranal
- Seproxetine
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal), tetronal, trional)
- Terpenoids (e.g., borneol)
- Topiramate
- Valerian constituents (e.g., isovaleric acid, isovaleramide, valerenic acid, valerenol)
|
|---|
|
 | Original source: https://en.wikipedia.org/wiki/Lofendazam. Read more |



