(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description
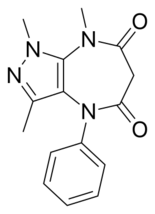
Zomebazam Identifiers
1,3,8-trimethyl-4-phenylpyrazolo[3,4-b ][1,4]diazepine-5,7-dione
CAS Number PubChem CID ChemSpider UNII Chemical and physical data Formula C 15 H 16 N 4 O 2 Molar mass −1 3D model (JSmol )
O=C1N(c2c(N(C(=O)C1)C)n(nc2C)C)c3ccccc3
InChI=1S/C15H16N4O2/c1-10-14-15(18(3)16-10)17(2)12(20)9-13(21)19(14)11-7-5-4-6-8-11/h4-8H,9H2,1-3H3
N Key:BFWACMHTIVUWJS-UHFFFAOYSA-N
N N Y (what is this?) (verify)
Zomebazam [1] anxiolytic properties. It is structurally related to razobazam and zometapine .[2]
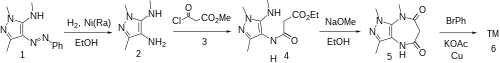
Synthesis The catalytic hydrogenation of N,2,5-trimethyl-4-phenyldiazenylpyrazol-3-amine CID:136203602 (1 ) over Raney Nickel gives 4-amino-1,3-dimethyl-5-methylaminopyrazole, CID:10219477 (2 ). Treatment with methyl malonyl chloride [37517-81-0] (3 ) gives 4-a-ethoxycarbonylacetylamino-1,3-dimethyl-5-methylaminopyrazole, CID:20561101 (4 ). Base catalyzed lactamization gives (5 ). The Goldberg reaction completes the synthesis Zomebazam (6 ).
See also References
↑ "4-Aryl-5,6,7,8-tetrahydropyrazolo(3,4-B)-(1,5)diazepine-1H,4H-5,7-diones and medicaments containing same" US patent 3558605
↑ "Zomebazam" . psychotropics.dk. 2003. http://www.psychotropics.dk/moleculeView/default.aspx?ID=1480&Catalogtype=A&ChapterID=237&Thissortorder=2 . ↑ "Direkte N-Arylierung von Amiden: Eine Verbesserung der Goldberg-Reaktion.". Synthesis 1985 (9): 856–560. 1985. doi :10.1055/s-1985-31364 . ↑ Rackur G, Hoffmann I, US patent 4302468 , issued 1981, assigned to Hoechst Aktiengesellschaft
Alcohols Barbiturates Benzodiazepines Carbamates Flavonoids Imidazoles Kava constituentsMonoureides Neuroactive steroids Nonbenzodiazepines Phenols Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases Others/unsorted
3-Hydroxybutanal α-EMTBL AA-29504 Avermectins (e.g., ivermectin )Bromide compounds (e.g., lithium bromide , potassium bromide , sodium bromide) Carbamazepine Chloralose Chlormezanone Clomethiazole DEABL Dihydroergolines (e.g., dihydroergocryptine , dihydroergosine , dihydroergotamine , ergoloid (dihydroergotoxine) )DS2 Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )Fluoxetine Flupirtine Hopantenic acid Lanthanum Lavender oil Lignans (e.g., 4-O-methylhonokiol , honokiol , magnolol , obovatol )Loreclezole Menthyl isovalerate (validolum) Monastrol Niacin Nicotinamide (niacinamide) Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal Seproxetine Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) , tetronal , trional )Terpenoids (e.g., borneol )Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide , valerenic acid , valerenol )
Original source: https://en.wikipedia.org/wiki/Zomebazam. Read more