Chemistry:Pyrazolam
From HandWiki
Short description: Benzodiazepine
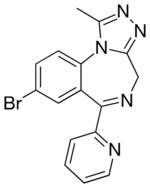 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Sublingual, rectal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 17 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H12BrN5 |
| Molar mass | 354.211 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pyrazolam (SH-I-04)[2] is a benzodiazepine derivative originally developed by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s.[3] It has since been "rediscovered" and sold as a designer drug since 2012.[4][5][6][7][8][9][excessive citations]
Pyrazolam has structural similarities to alprazolam[10] and bromazepam. Unlike other benzodiazepines, pyrazolam does not appear to undergo metabolism, instead being excreted unchanged in the urine.[4]
Legal status
United Kingdom
In the UK, pyrazolam has been classified as a Class C drug by section 5 of the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[11]
See also
References
- ↑ (customs (prohibited imports) regulations 1956 - schedule 4, 1956)
- ↑ "A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model". International Journal of Medicinal Chemistry 2015: 430248. 2015. doi:10.1155/2015/430248. PMID 26682068.
- ↑ Sternbach LH, Walser A, "Preparation of triazolo benzodiazepines and novel compounds", US patent 3954728, issued 4 May 1976, assigned to Hoffmann La Roche Inc.
- ↑ 4.0 4.1 "Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine". Forensic Toxicology 31 (2): 263–271. July 2013. doi:10.1007/s11419-013-0187-4.
- ↑ "Designer benzodiazepines: A new challenge". World Psychiatry 14 (2): 248. June 2015. doi:10.1002/wps.20236. PMID 26043347.
- ↑ "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis 9 (4): 640–645. April 2017. doi:10.1002/dta.2003. PMID 27366870.
- ↑ "Blood concentrations of new designer benzodiazepines in forensic cases". Forensic Science International 268: 35–38. November 2016. doi:10.1016/j.forsciint.2016.09.006. PMID 27685473.
- ↑ "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis 10 (8): 1258–1269. March 2018. doi:10.1002/dta.2387. PMID 29582576. https://rke.abertay.ac.uk/en/publications/527a634d-decc-4d3a-bdca-08659bb13ed6.
- ↑ "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology 40 (2): 349–356. July 2022. doi:10.1007/s11419-022-00616-y. PMID 36454409.
- ↑ "6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity". Journal of Medicinal Chemistry 14 (11): 1078–1081. November 1971. doi:10.1021/jm00293a015. PMID 5165540.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2017". legislation.gov.uk. http://www.legislation.gov.uk/uksi/2017/634/contents/made.
 |

