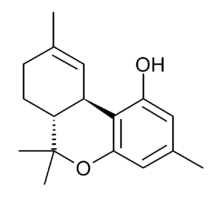
Chemistry:Tetrahydrocannabiorcol
From HandWiki
Short description: Chemical compound
 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H22O2 |
| Molar mass | 258.361 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Δ9-Tetrahydrocannabiorcol (Δ9-THCC, (C1)-Δ9-THC) is a phytocannabinoid found in Cannabis pollen.[1] It is a homologue of THC and THCV with the alkyl side chain replaced by a smaller methyl group. Unlike THC and THCV, THCC has negligible affinity for the CB1 and CB2 cannabinoid receptors because of the smaller methyl group and does not have psychoactive effects as a result, but conversely it is significantly more potent than THC or THCV as an activator of the TRPA1 calcium channel which plays an important role in pain perception,[2] and it has been shown to produce analgesic effects via activation of spinal TRPA1 channels. [3][4] THCC was studied by Roger Adams as early as 1942.[5]
See also
References
- ↑ "Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L". Phytochemical Analysis 16 (1): 45–8. 2005. doi:10.1002/pca.809. PMID 15688956.
- ↑ "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry 8: 17–39. 2016. doi:10.4137/PMC.S32171. PMID 27398024.
- ↑ "TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol". Nature Communications 2: 551. November 2011. doi:10.1038/ncomms1559. PMID 22109525. Bibcode: 2011NatCo...2..551A.
- ↑ "Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain". Proceedings of the National Academy of Sciences of the United States of America 111 (47): 16901–6. November 2014. doi:10.1073/pnas.1412689111. PMID 25389312. Bibcode: 2014PNAS..11116901M.
- ↑ https://pubs.acs.org/doi/abs/10.1021/ja01255a061
 |

